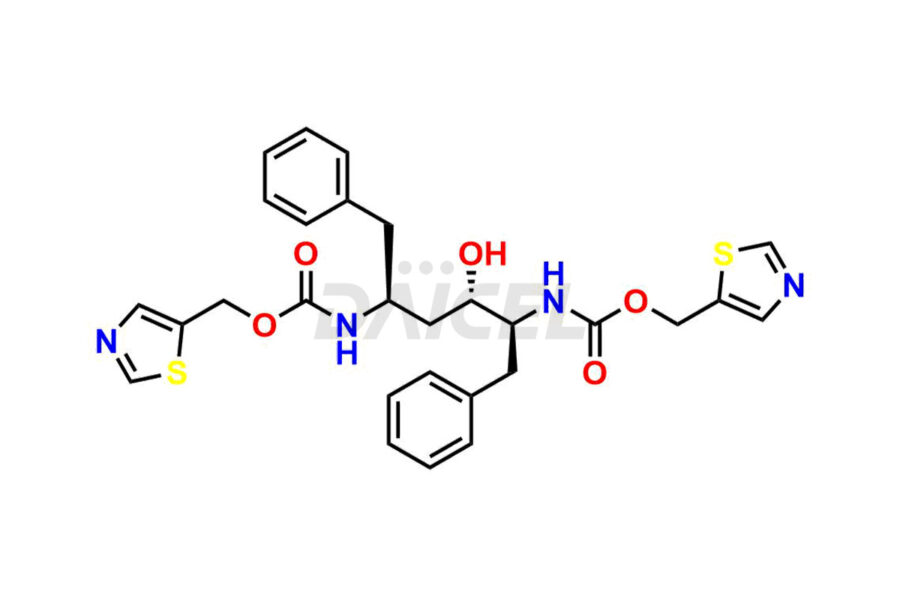
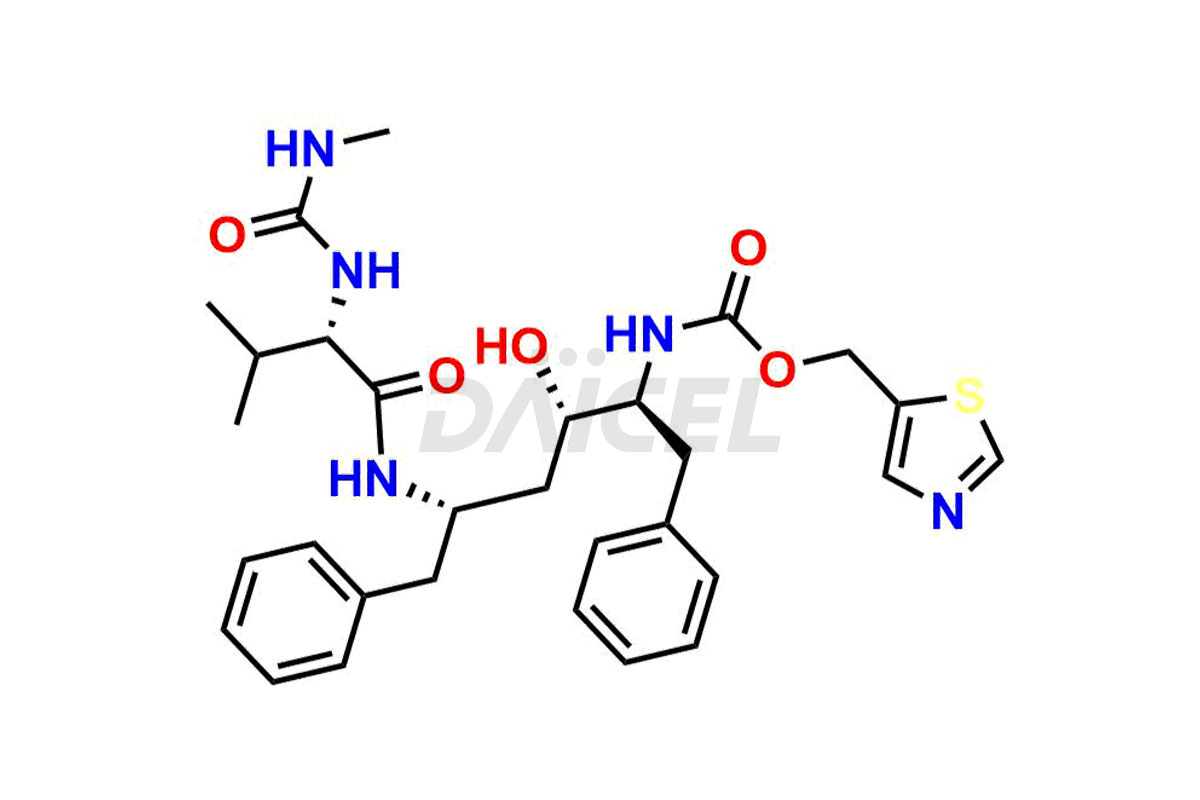
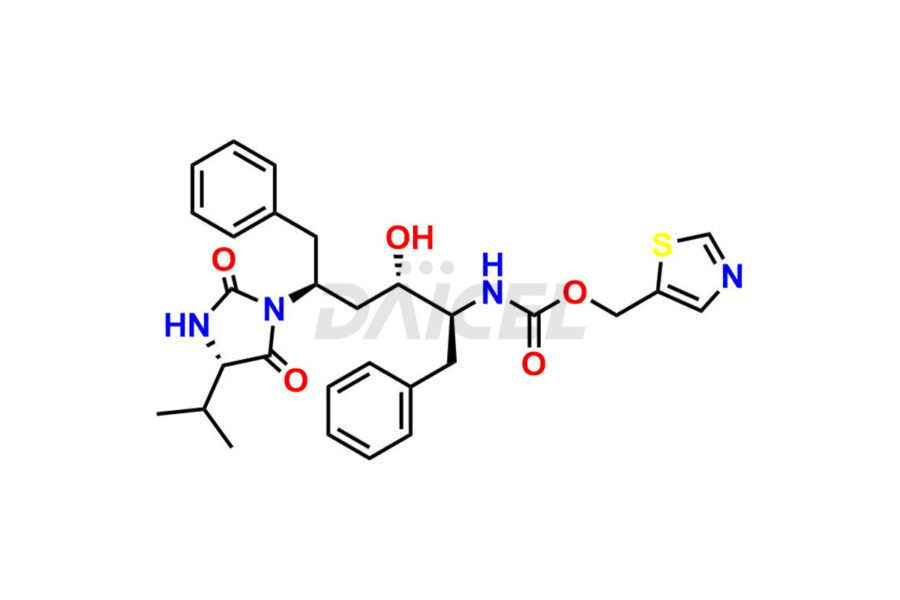
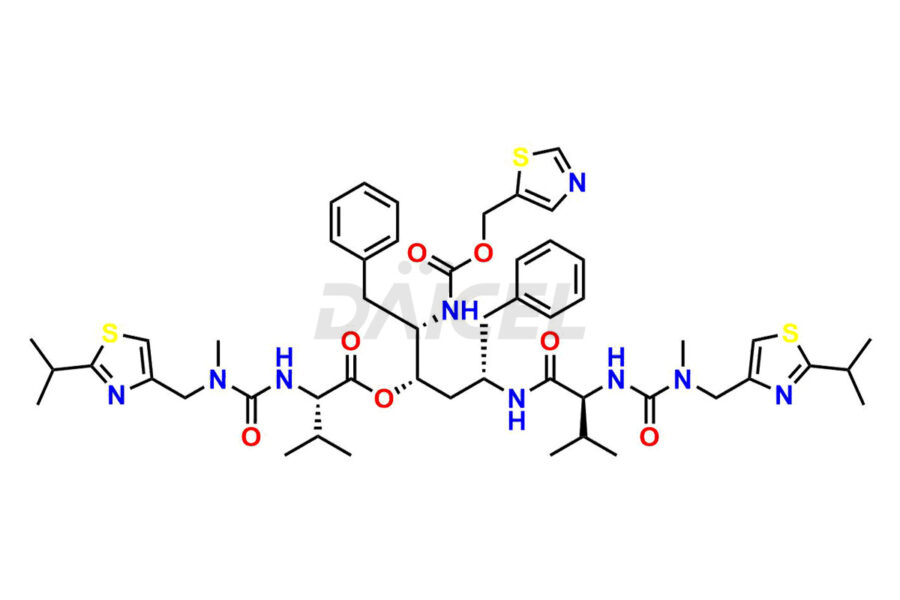
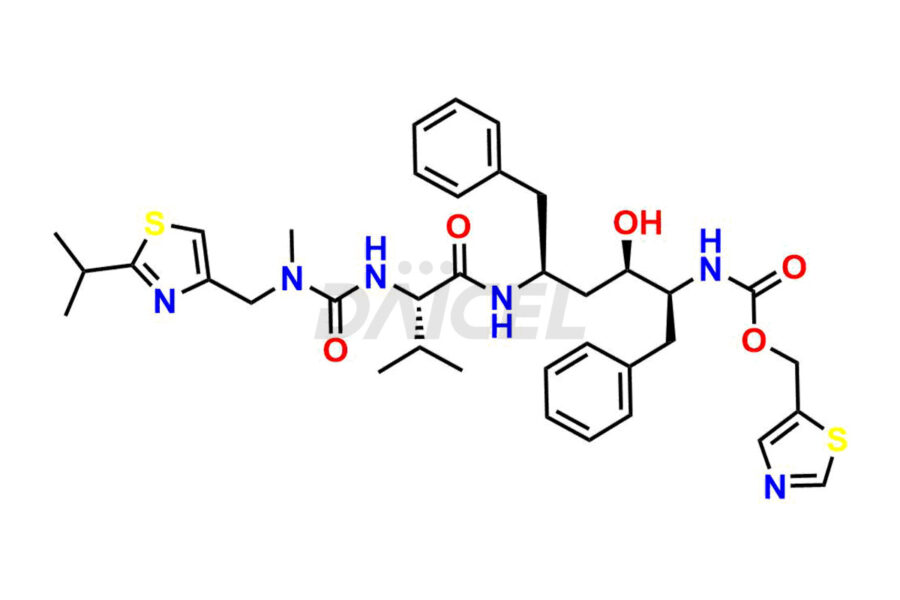
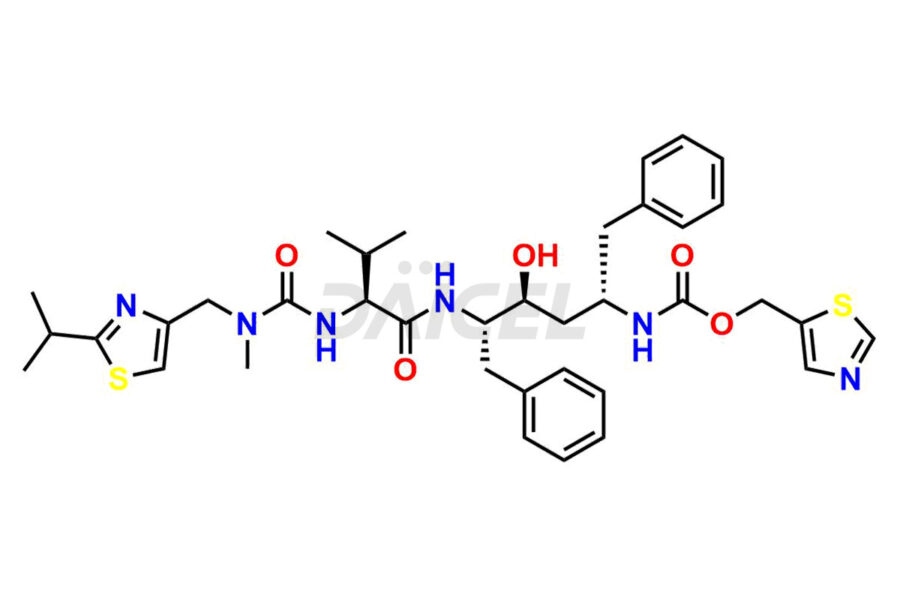
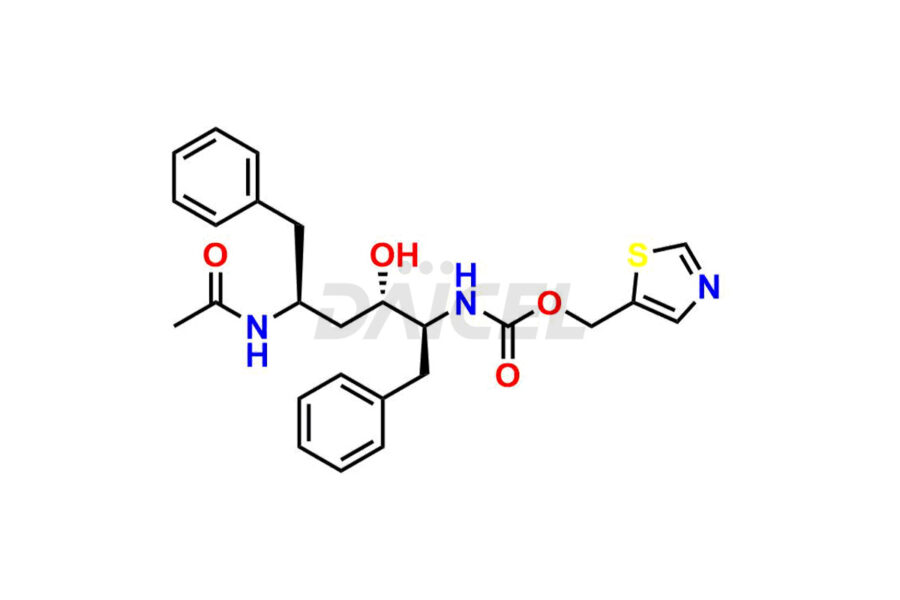
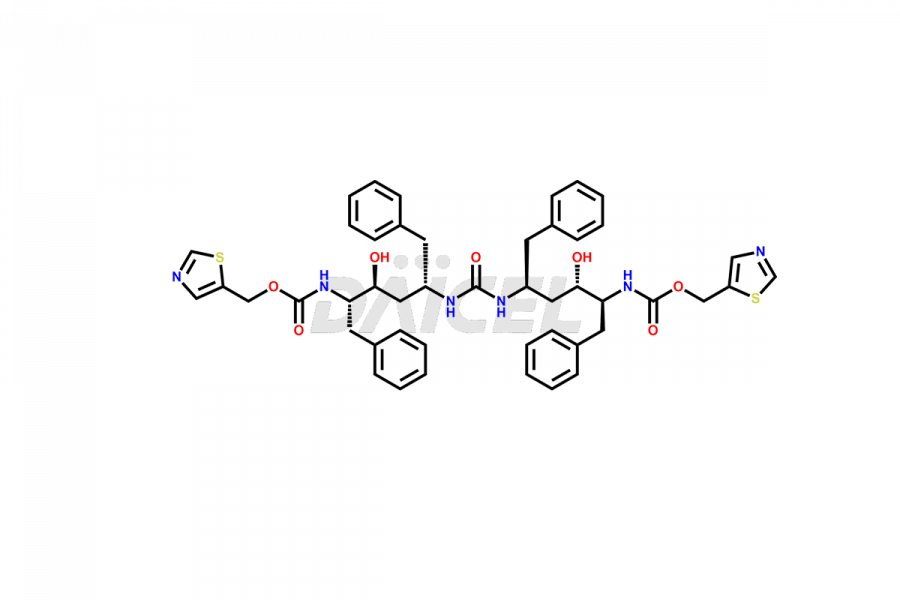
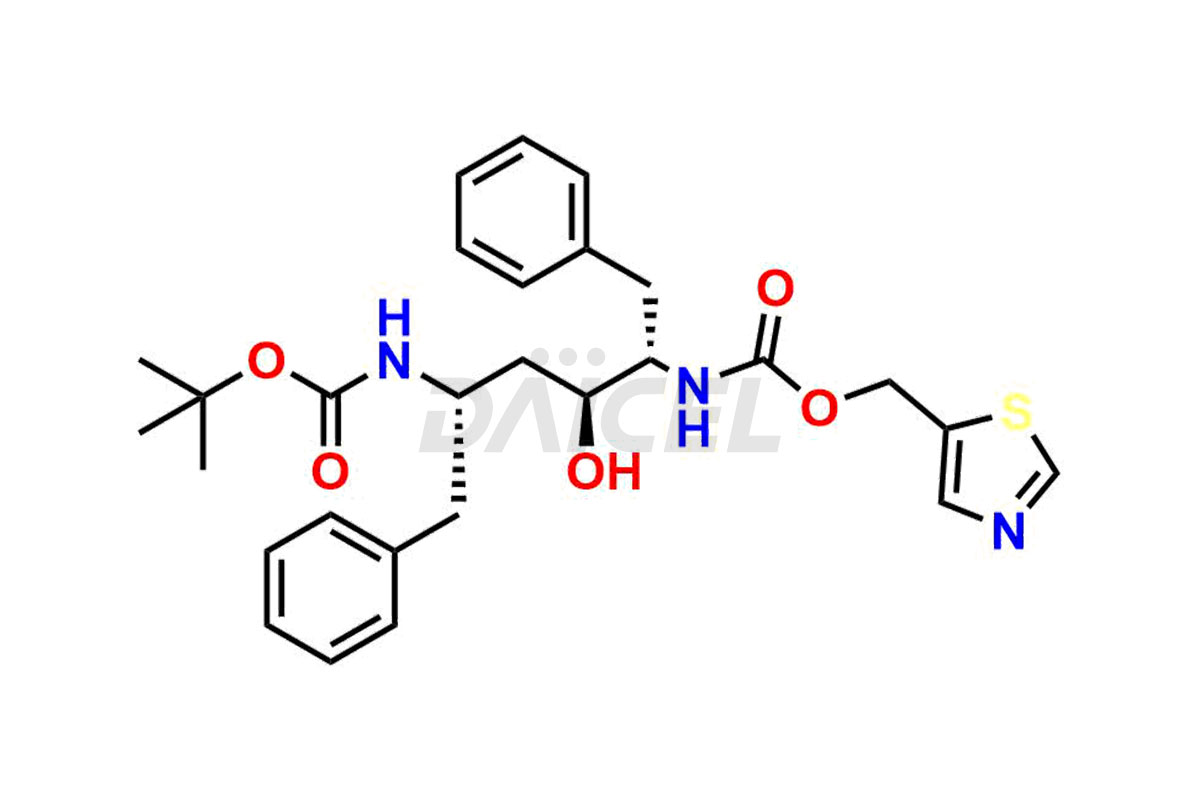
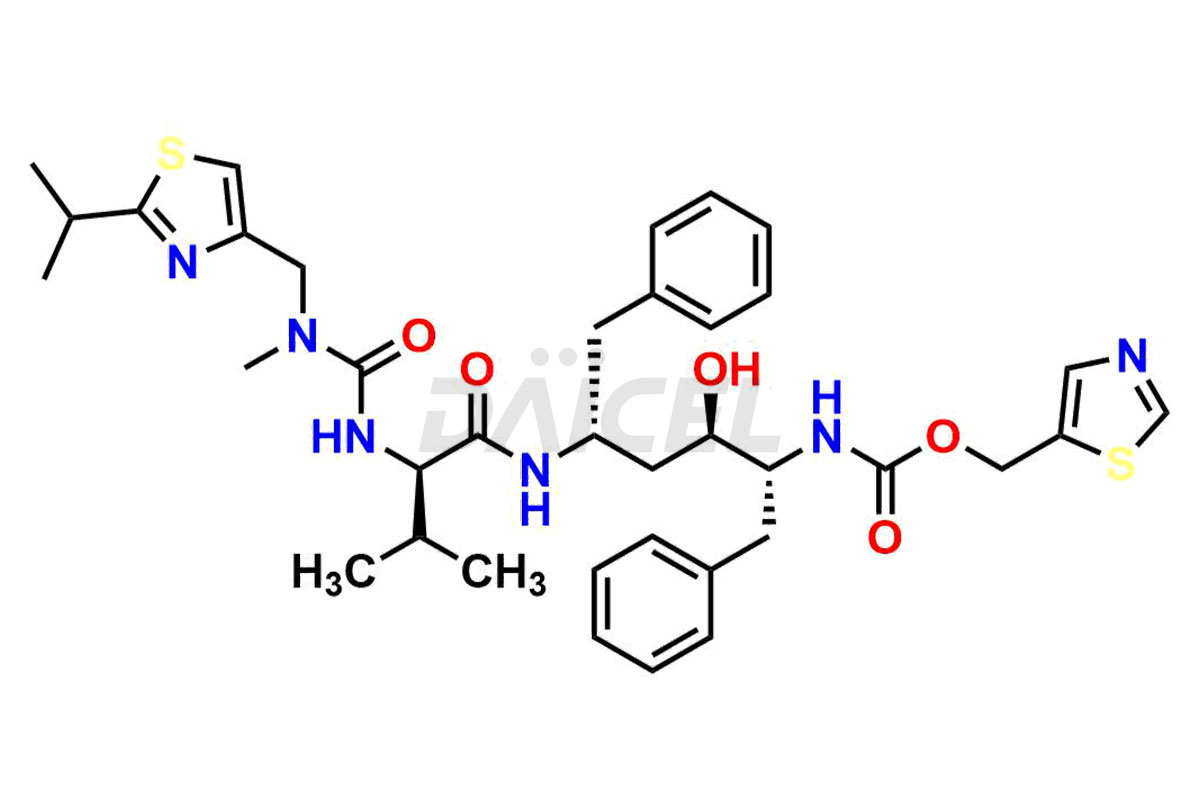
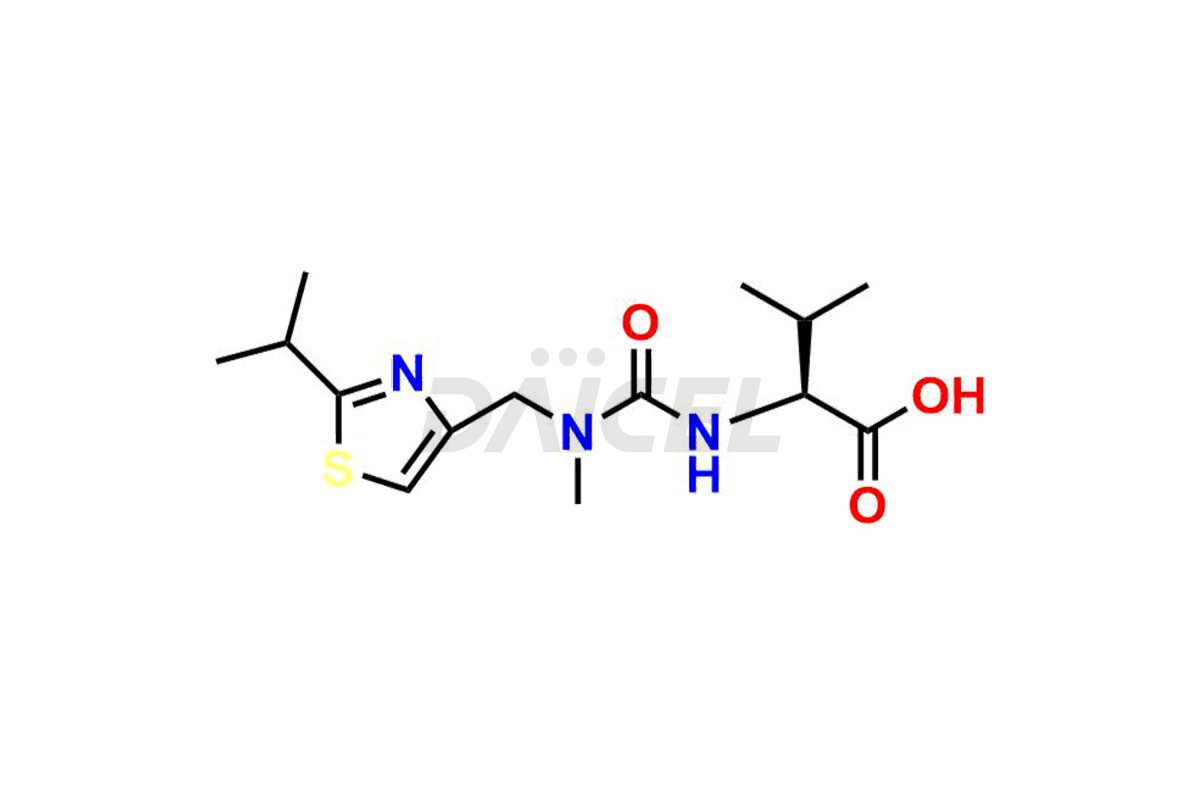
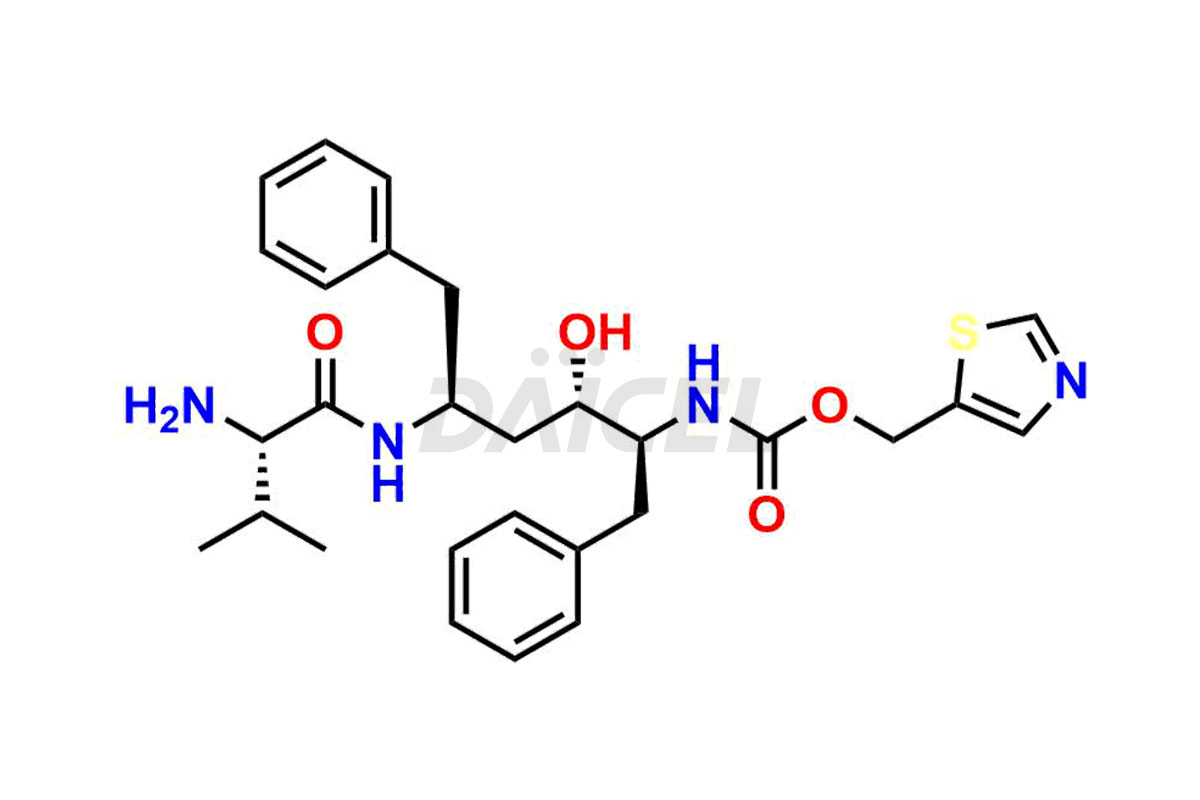
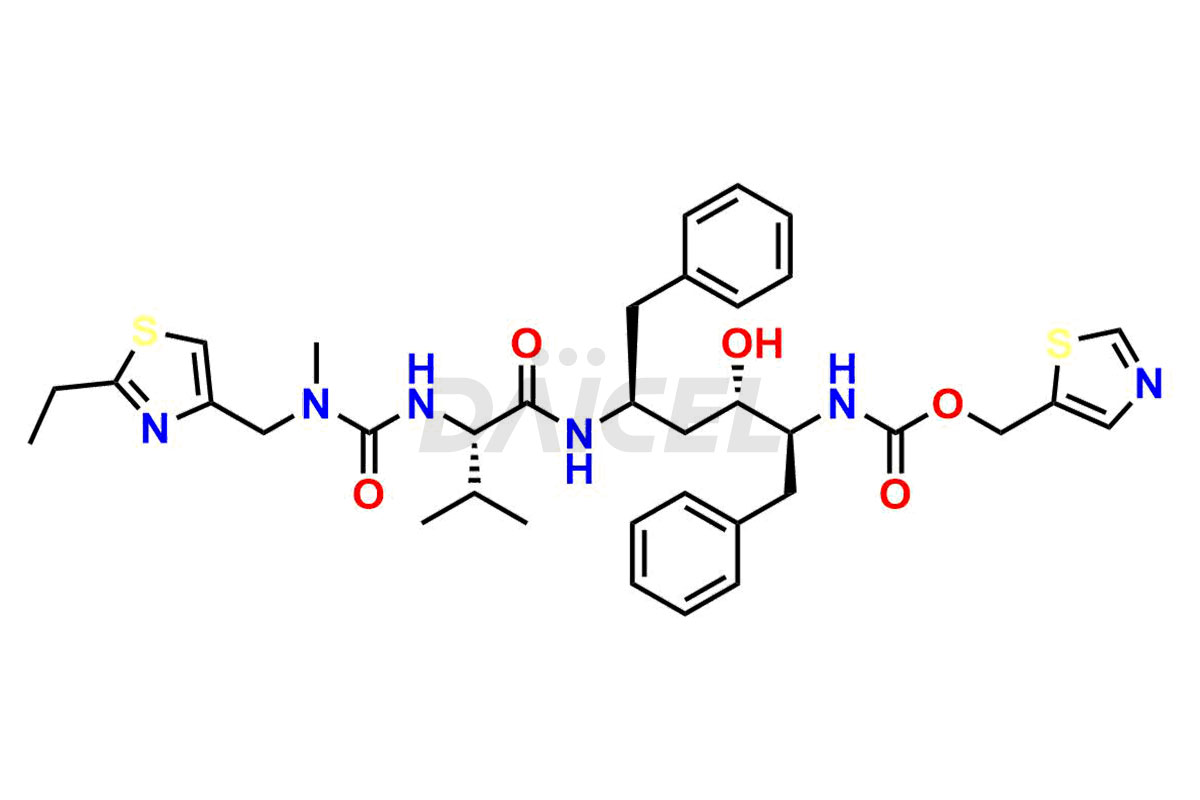
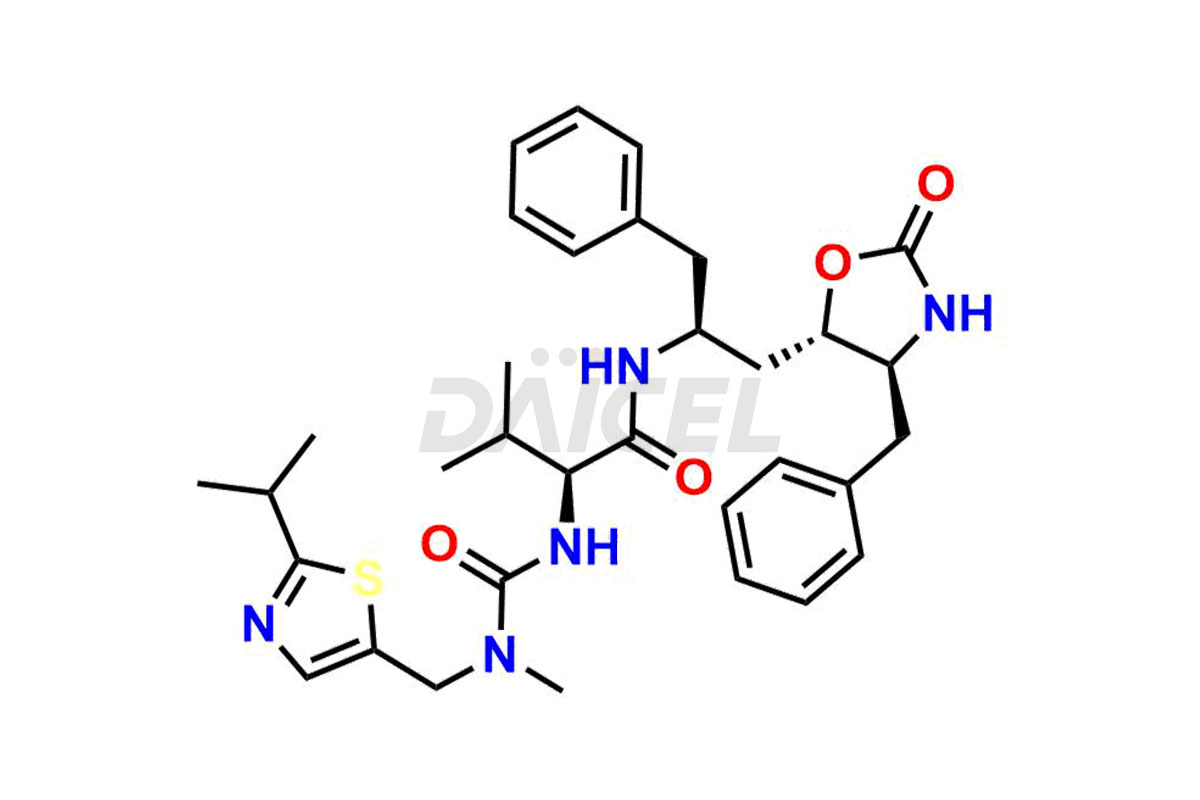
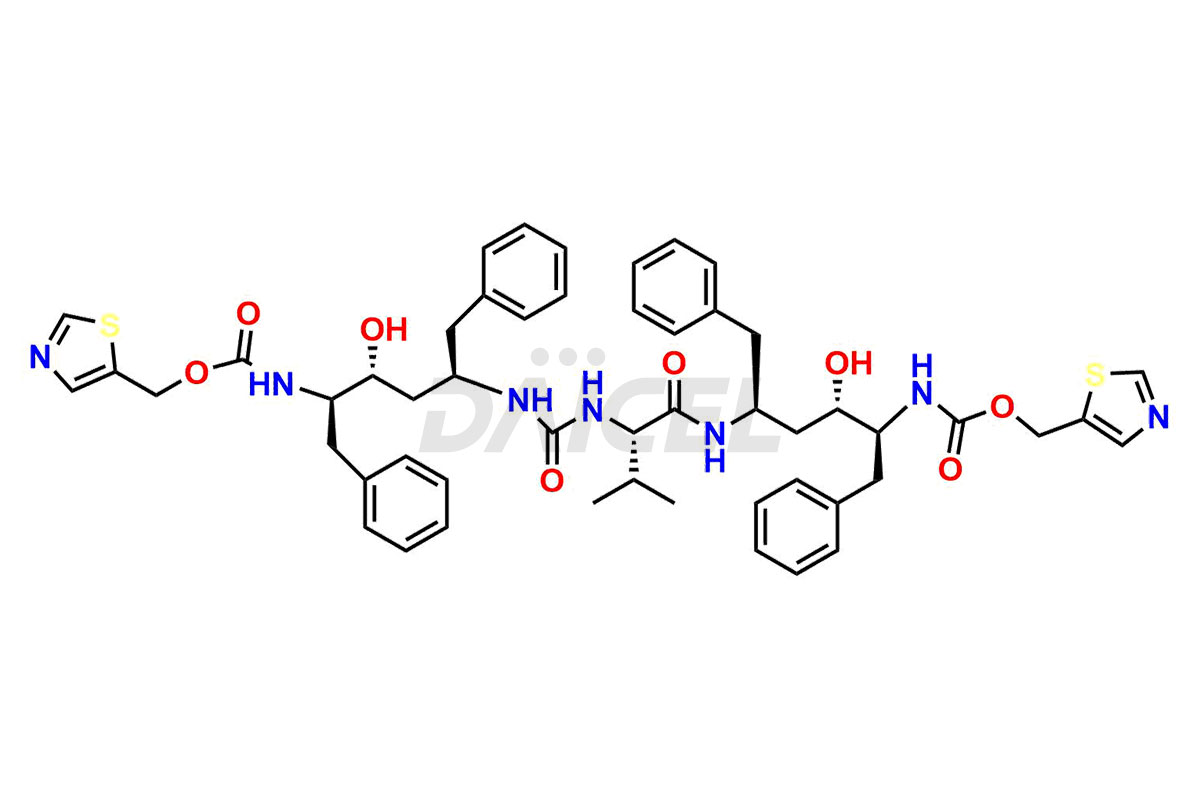
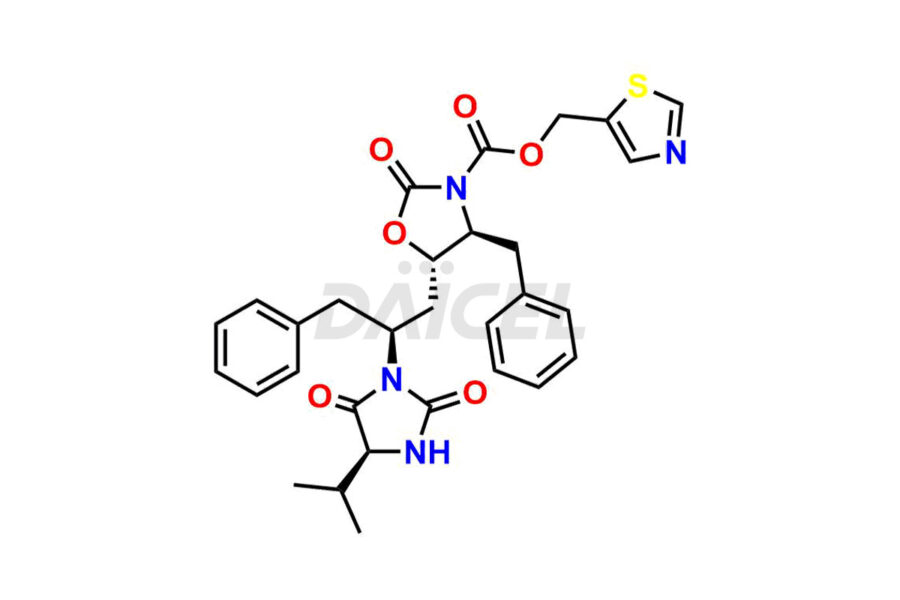
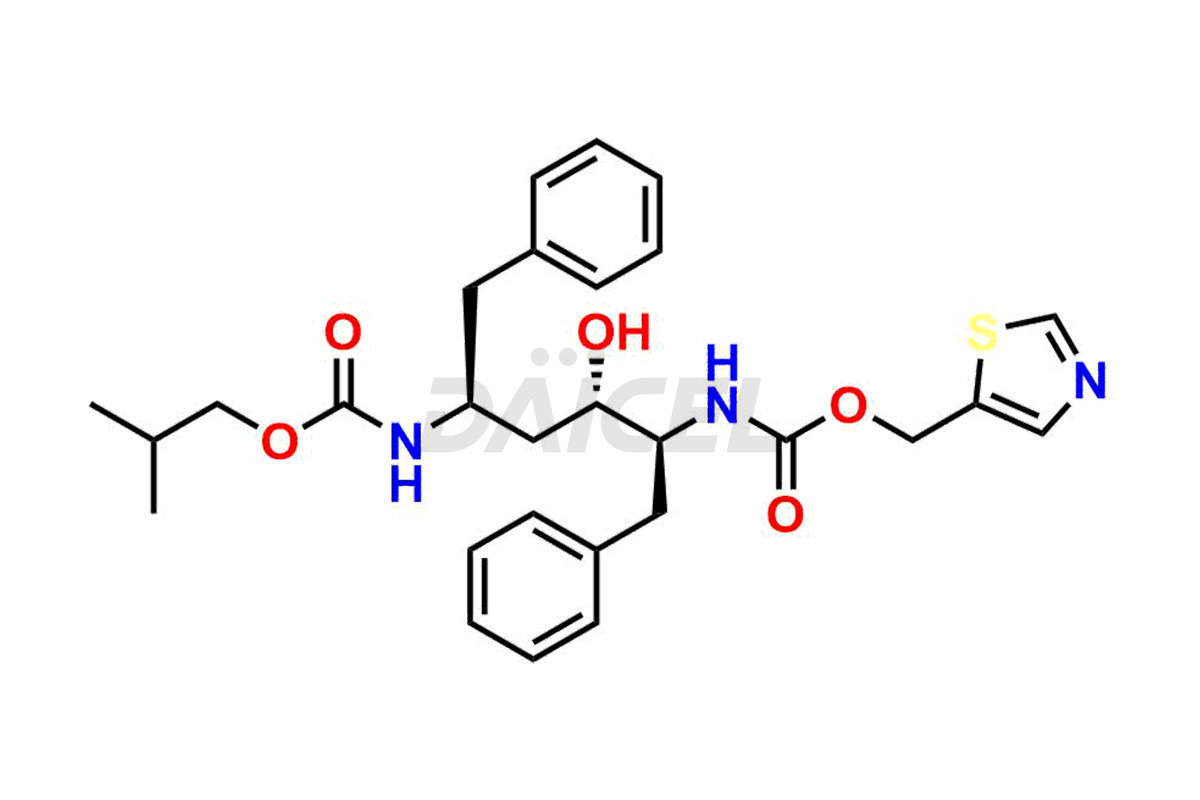
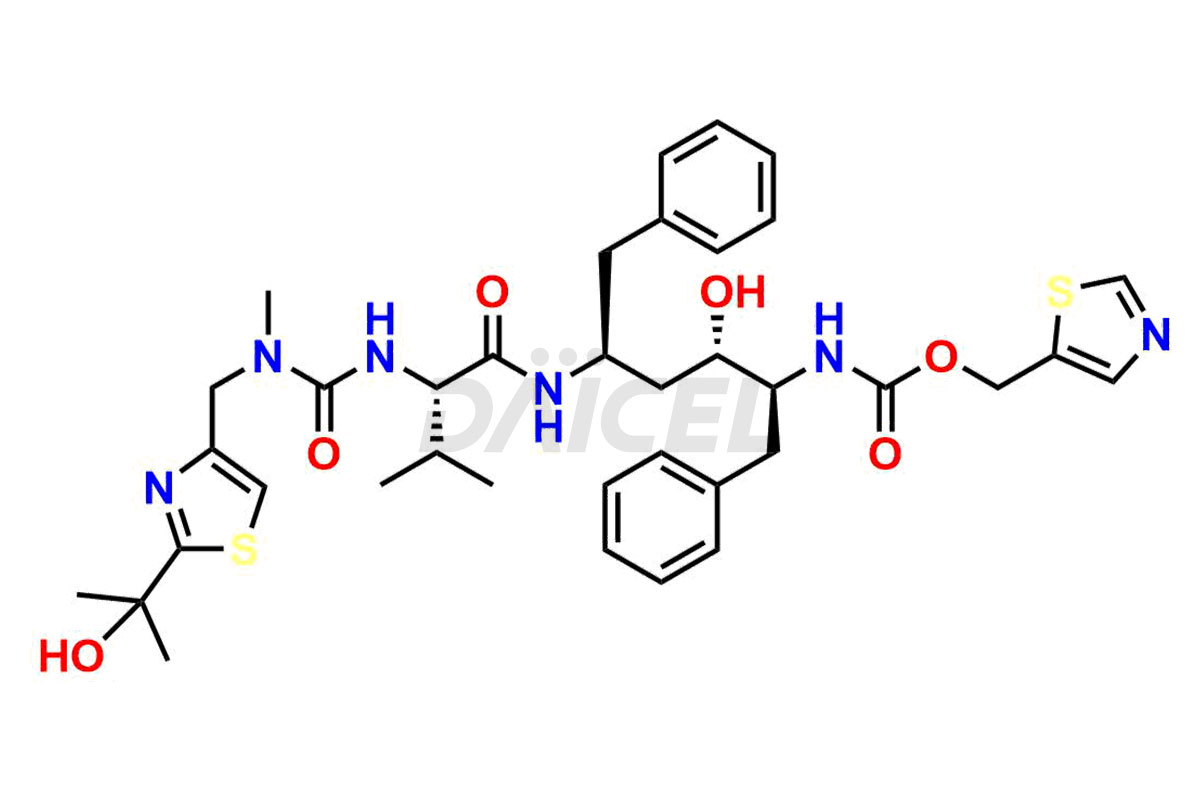
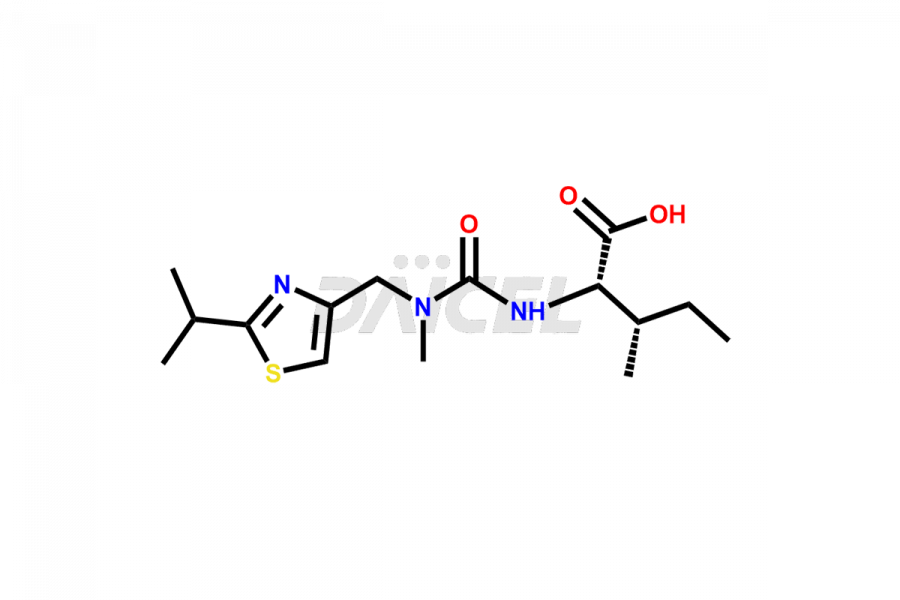
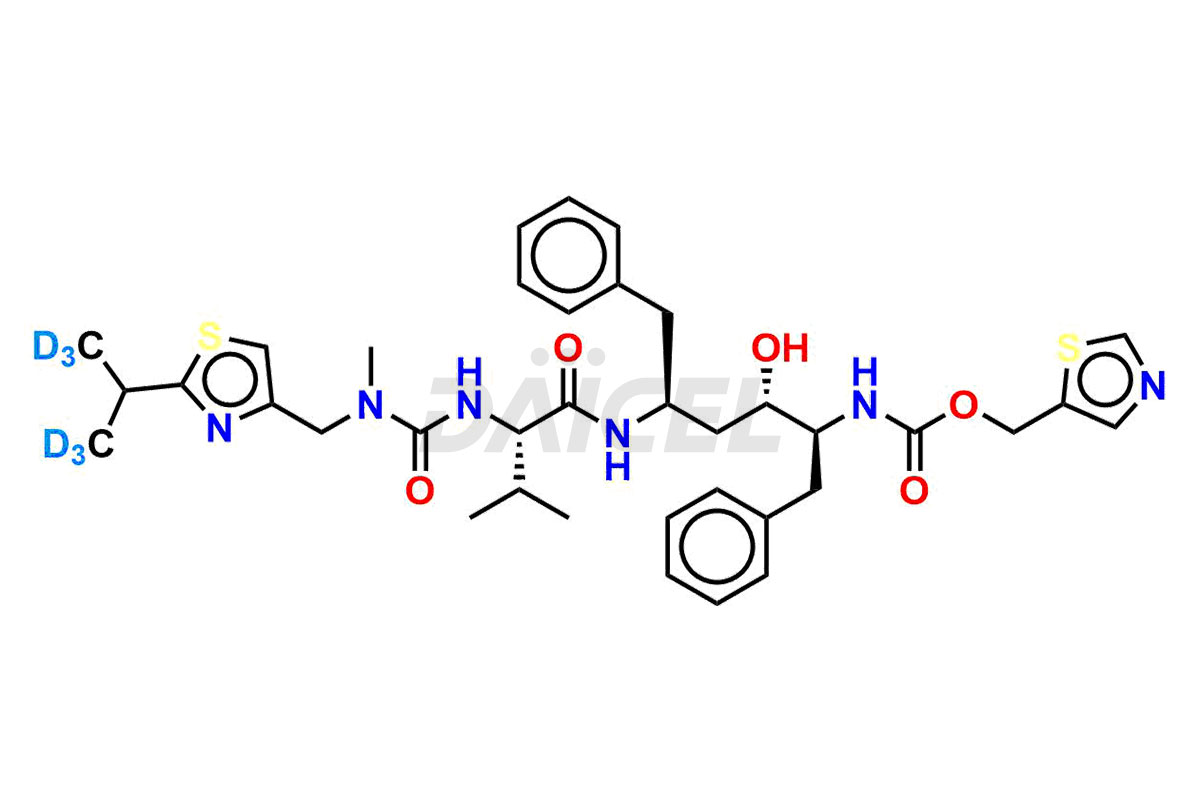
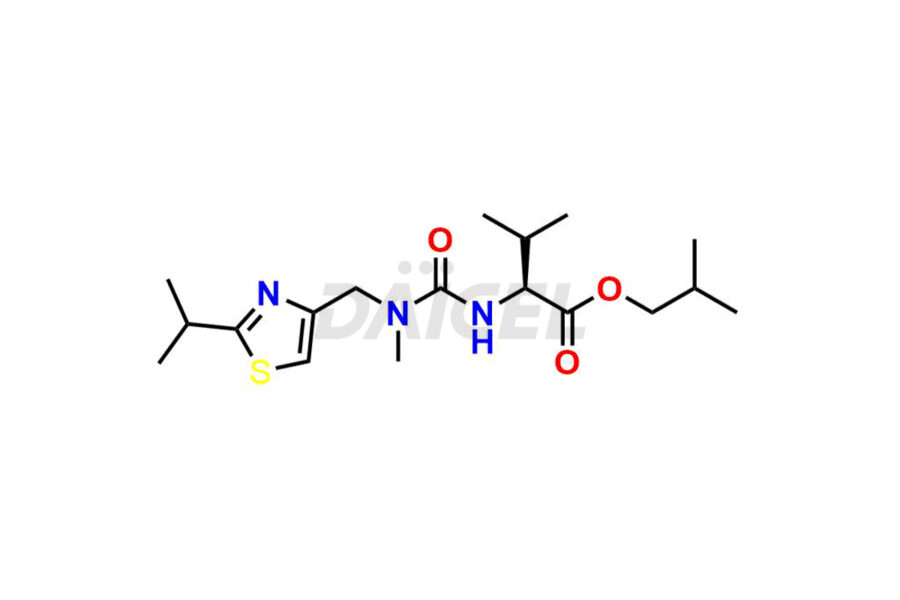
Ritonavir
General Information
Ritonavir Impurities and Ritonavir
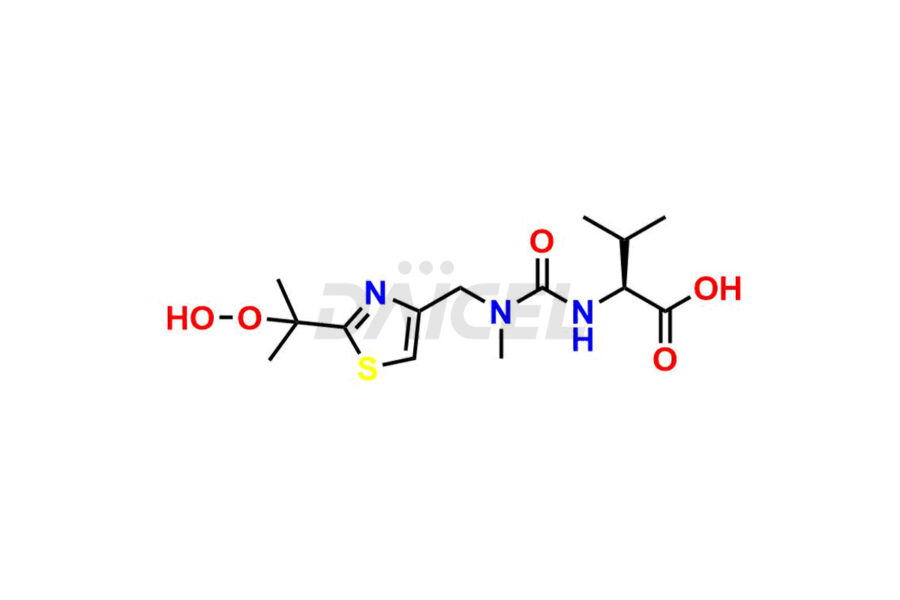
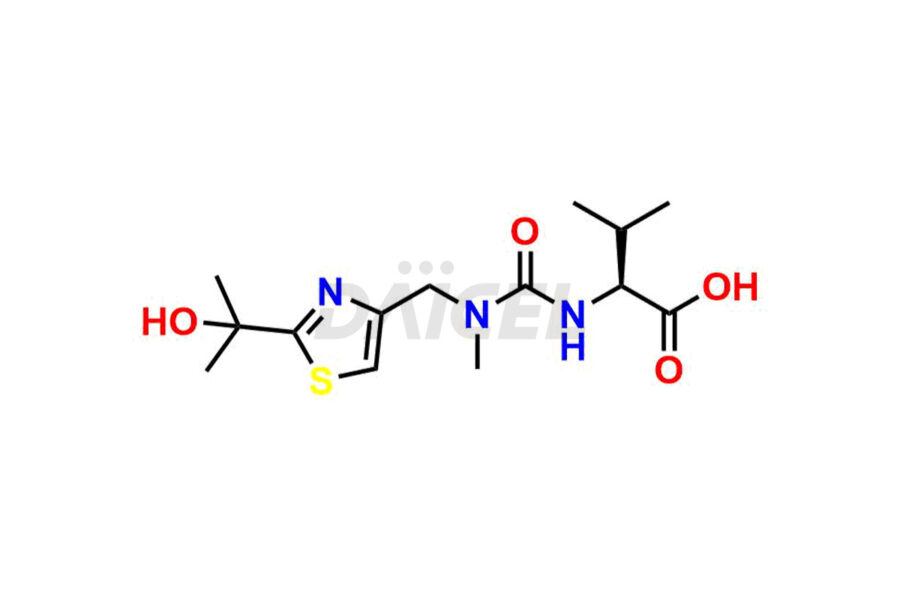
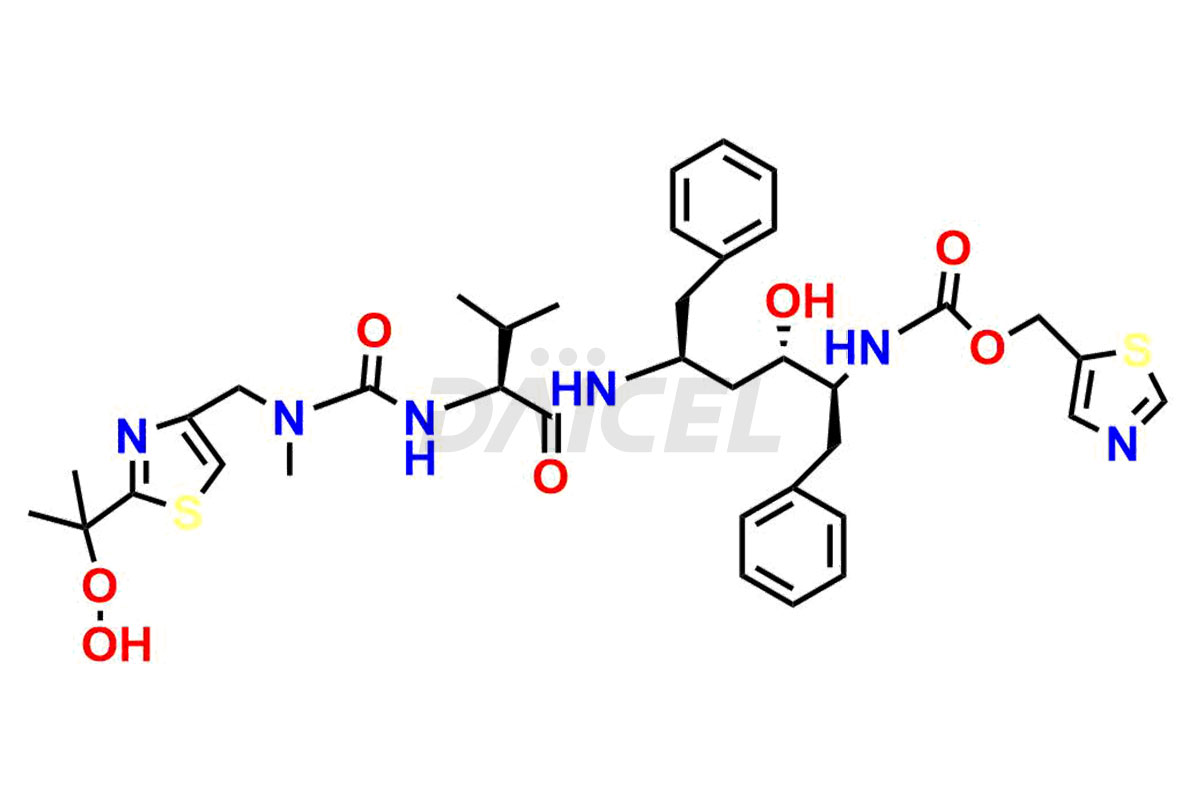
Daicel Pharma provides high-quality Ritonavir impurity standards, which include 2-Chloro NCT Impurity of Ritonavir, Ritonavir 3R-Epimer, Ritonavir Impurity K, Ritonavir Hydroxy Intermediate Impurity, Ritonavir Impurity G, Ritonavir Enantiomer, Ritonavir Impurity-E, Ritonavir EP Impurity L, and more. They are essential for Ritonavir effectiveness, safety, stability, and quality. Daicel Pharma can provide a custom synthesis for Ritonavir impurities and deliver them globally according to client requirements.
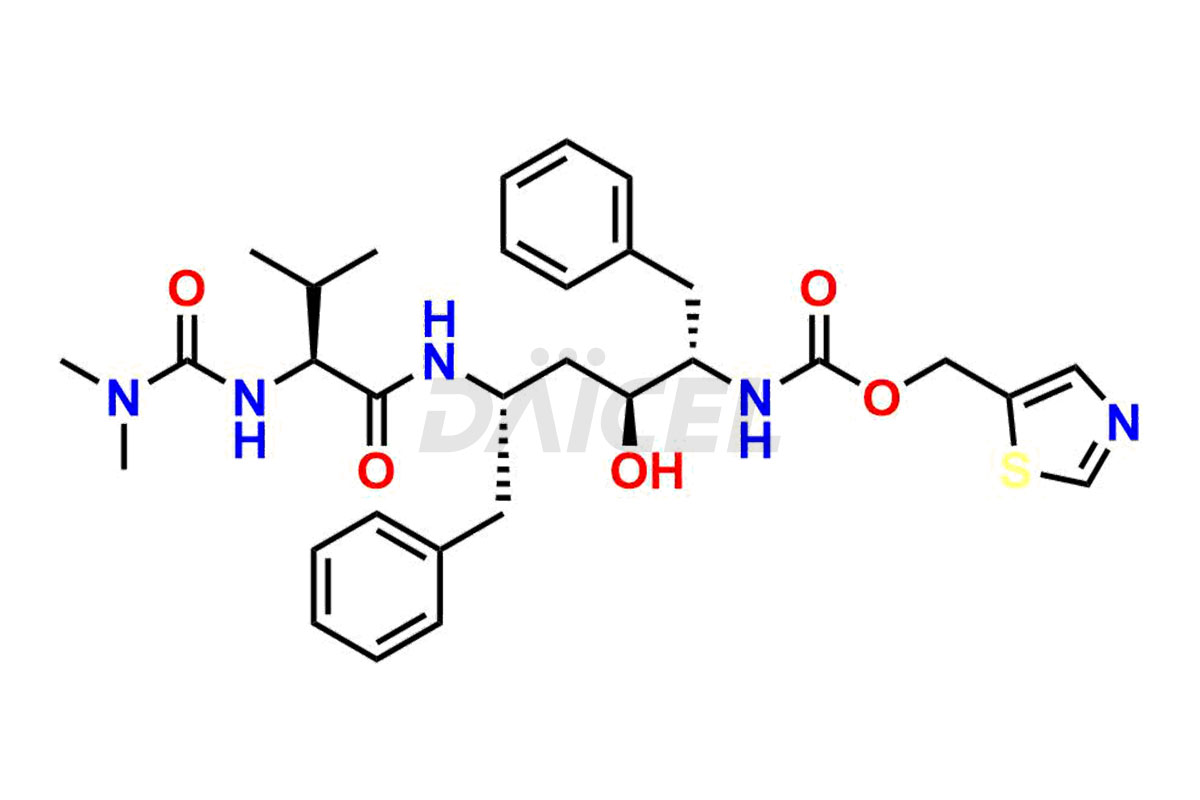
Ritonavir [CAS: 155213-67-5] is an L-valine derivative used to treat HIV infection and AIDS.
Ritonavir: Use and Commercial Availability
Ritonavir is an HIV protease inhibitor. It combines with other antiretroviral agents to treat HIV infection. The drug is available under the tradename of Norvir.
Ritonavir Structure and Mechanism of Action 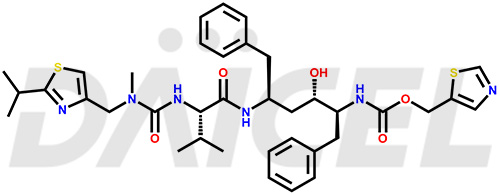
The chemical name of Ritonavir is (3S,4S,6S,9S)- 4-hydroxy-12-methyl-9-(1-methylethyl)-13-[2-(1-methylethyl)-4-thiazolyl]-8,11-dioxo-3,6-bis(phenylmethyl)- 2,7,10,12-Tetraazatridecanoic acid 5-thiazolylmethyl ester. Its chemical formula is C37H48N6O5S2, and its molecular weight is approximately 720.9 g/mol.
Ritonavir inhibits HIV-1 and HIV-2 proteases and leads to the production of non-infectious immature HIV particles.
Ritonavir Impurities and Synthesis
Ritonavir, an antiretroviral medication, may contain impurities impacting its efficacy and safety. Ritonavir impurities include related compounds, process-related, and potential impurities. They can arise from the synthetic process1, starting materials, or storage conditions. Ritonavir impurities require strict control and monitoring to assure drug safety, efficacy, and quality, and analytical procedures help identify, quantify, and characterize these impurities.
Daicel Pharma offers a Certificate of Analysis (CoA) for Ritonavir impurity standards which include 2-Chloro NCT Impurity of Ritonavir, Ritonavir 3R-Epimer, Ritonavir Impurity K, Ritonavir Hydroxy Intermediate Impurity, Ritonavir Impurity G, Ritonavir Enantiomer, Ritonavir Impurity-E, Ritonavir EP Impurity L, and more. Daicel Pharma’s cGMP-certified analytical laboratory provides this CoA and includes detailed characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. More characterization details, such as those for 13C-DEPT, are provided on request. Daicel Pharma offers Ritonavir impurities and labeled compounds for testing the efficacy of generic Ritonavir. We can also give Ritonavir-D6, a deuterium-labeled Ritonavir standard for bioanalytical research and Bioavailability/Bioequivalence (BA/BE) studies.
References
FAQ's
References
- Kempf, Dale J.; Norbeck, Daniel W.; Sham, Hing Leung; Zhao, Chen; Sowin, Thomas J.; Reno, Daniel S.; Haight, Anthony R.; Cooper, Arthur J., Retroviral Protease Inhibiting Compounds, Abbott Laboratories, United States, EP674513B1, September 25, 1996
- Marsh, Kennan C.; Eiden, Erin; McDonald, Edith, Determination of ritonavir, a new HIV protease inhibitor, in biological samples using reversed-phase high-performance liquid chromatography, Journal of Chromatography B: Biomedical Sciences and Applications, Volume: 704, Issue: 1 + 2, Pages: 307-313, 1997
Frequently Asked Questions
How are Ritonavir impurities formed?
Ritonavir impurities form during the synthetic process from starting materials or as degradation products due to various factors such as reaction conditions, impurities in raw materials, or storage conditions.
Are there any specific acceptance criteria for Ritonavir impurities?
Specific acceptance criteria are established for Ritonavir impurities to ensure their levels remain within defined limits and according to regulatory guidelines.
Are Ritonavir impurities related to the quality or efficiency of the drug?
Ritonavir impurities can be related to the quality and efficiency of the drug.
What are the temperature conditions required to store Ritonavir impurities?
Ritonavir impurities should be stored at a controlled room temperature between 2-8°C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.