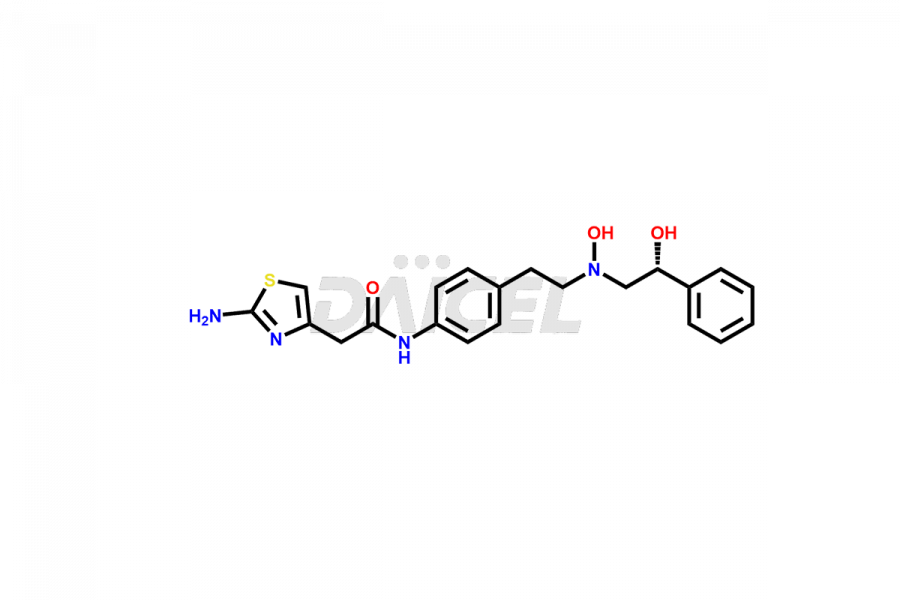
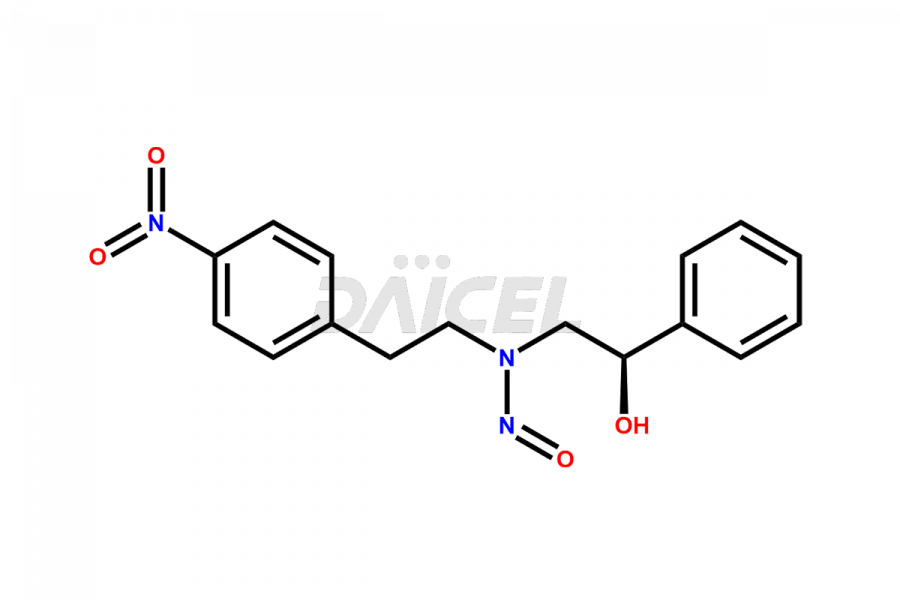
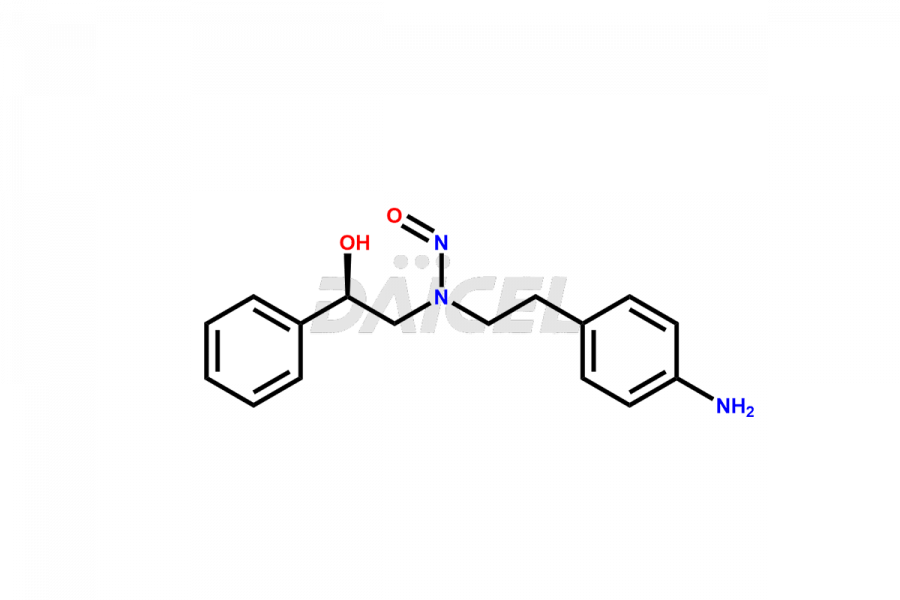
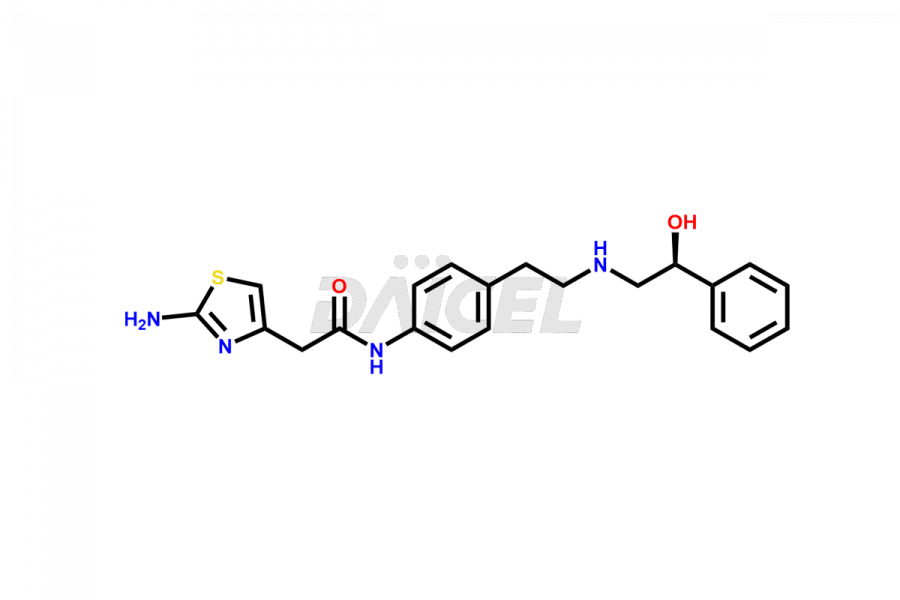
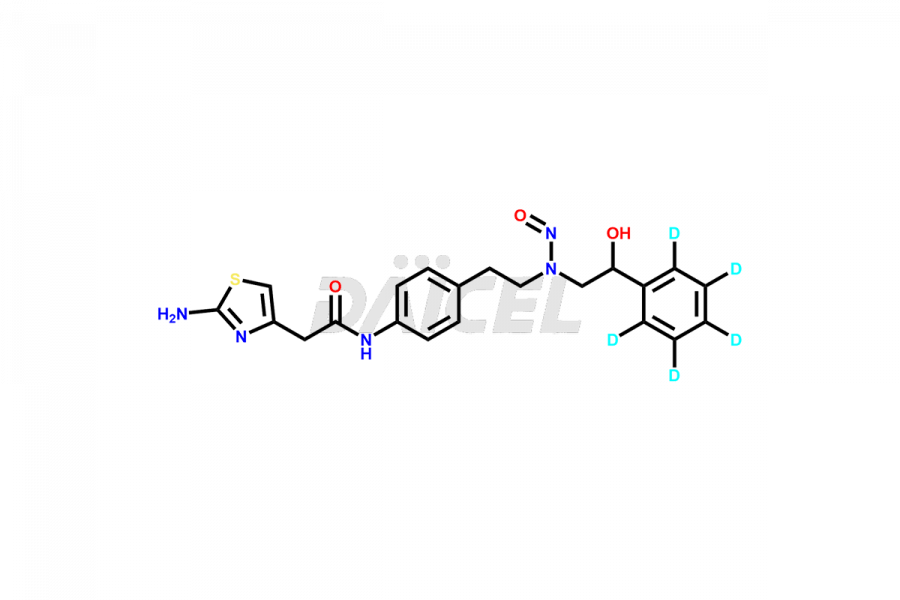
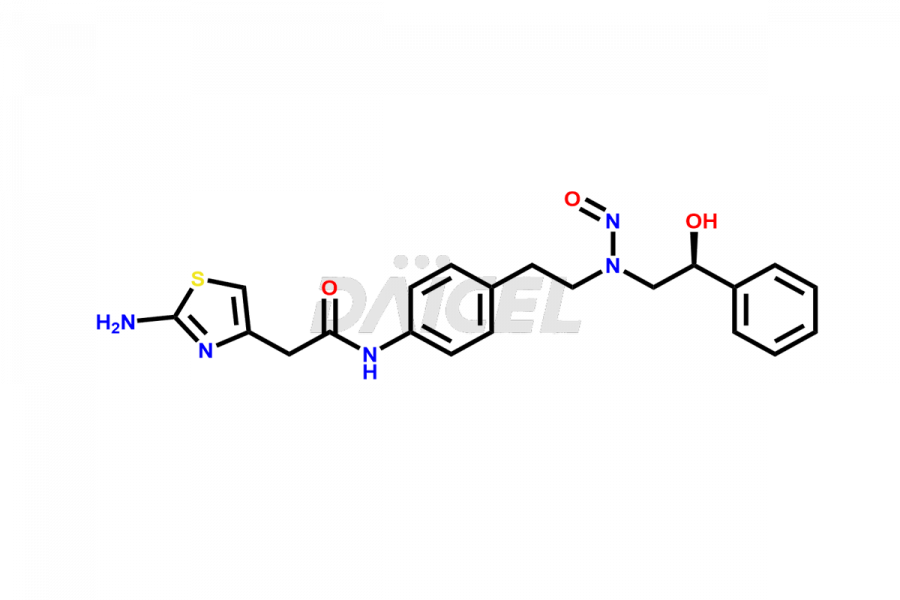
Mirabegron
General Information
Mirabegron Impurities and Mirabegron
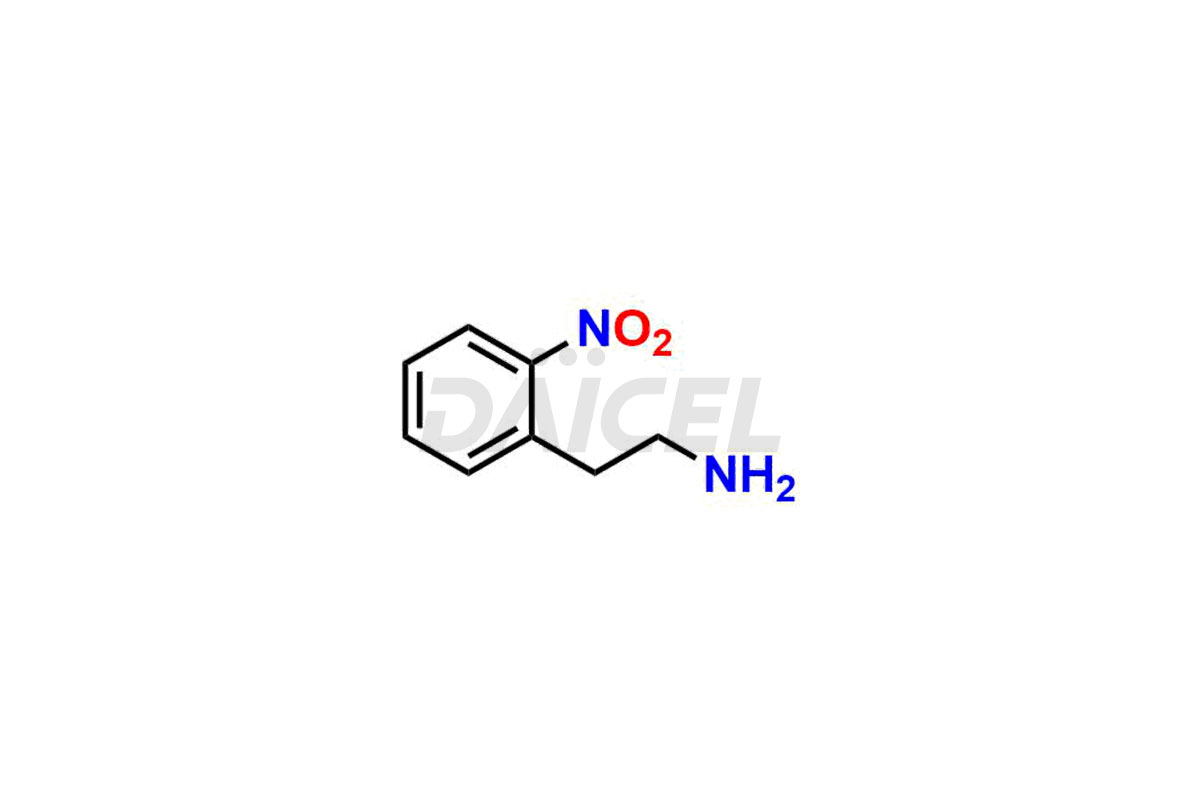
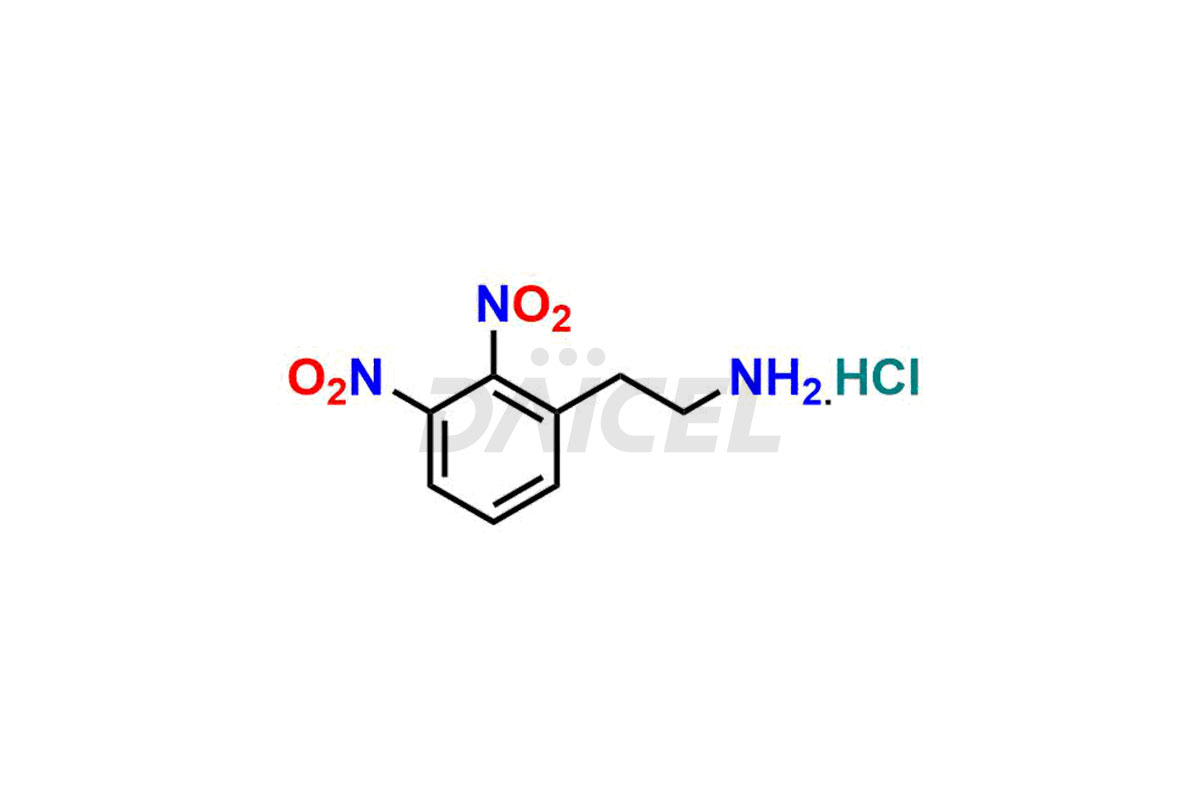
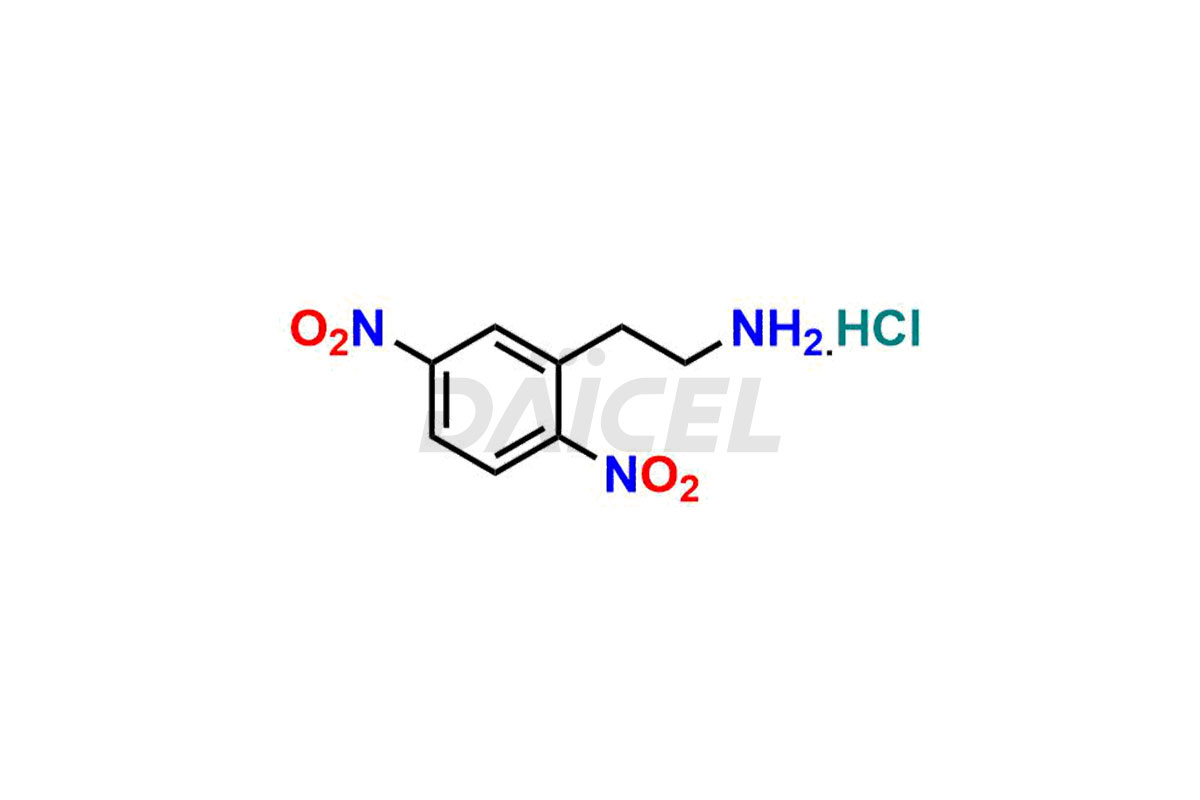
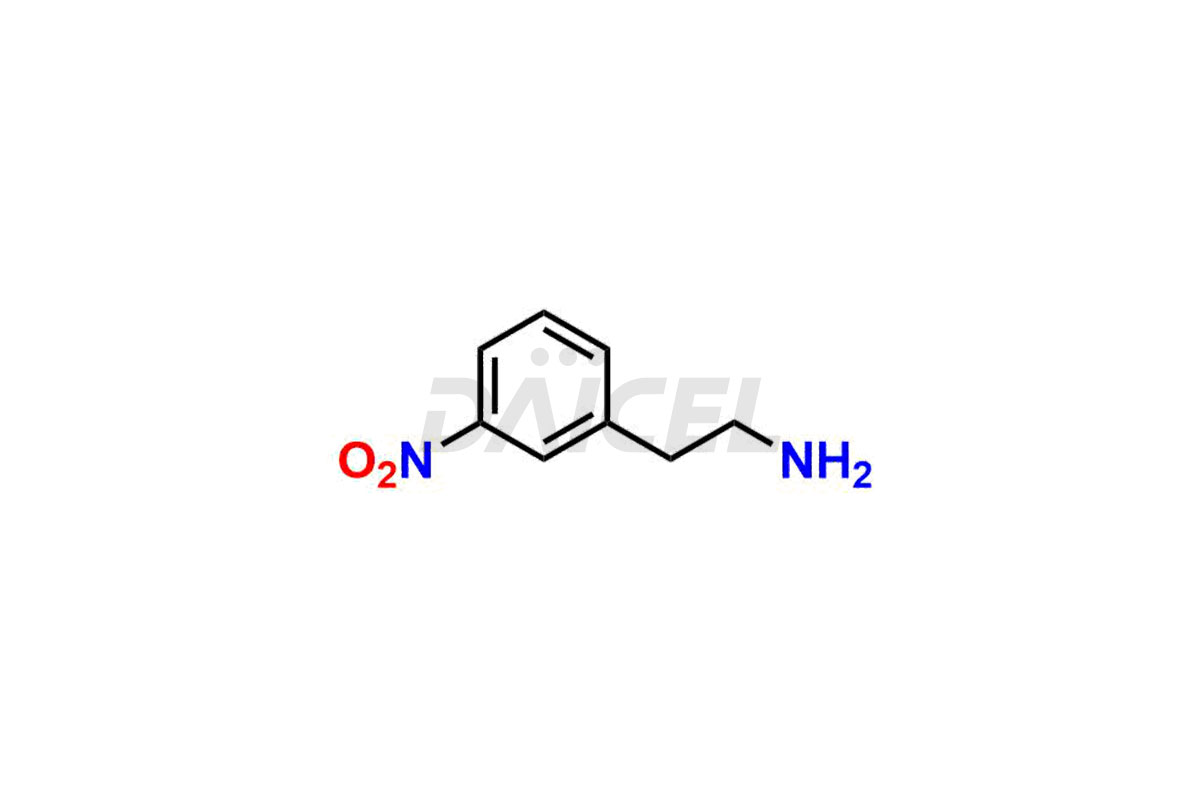
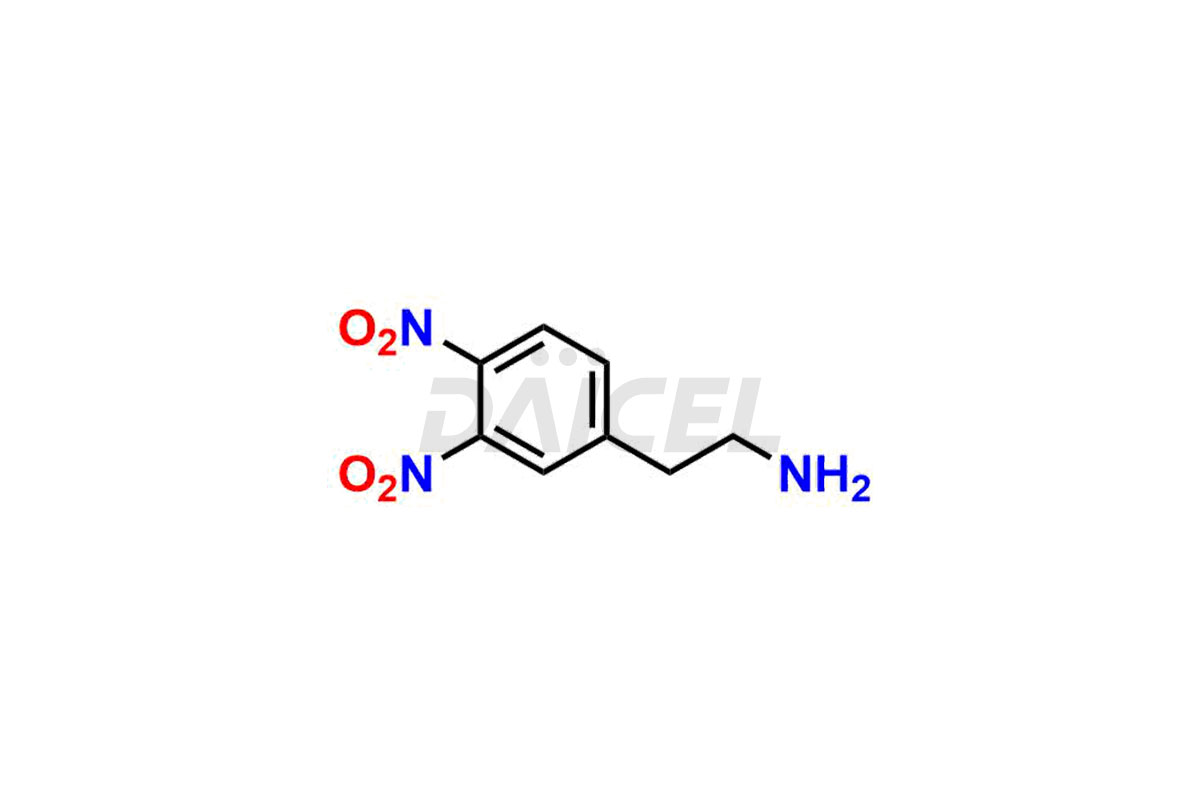
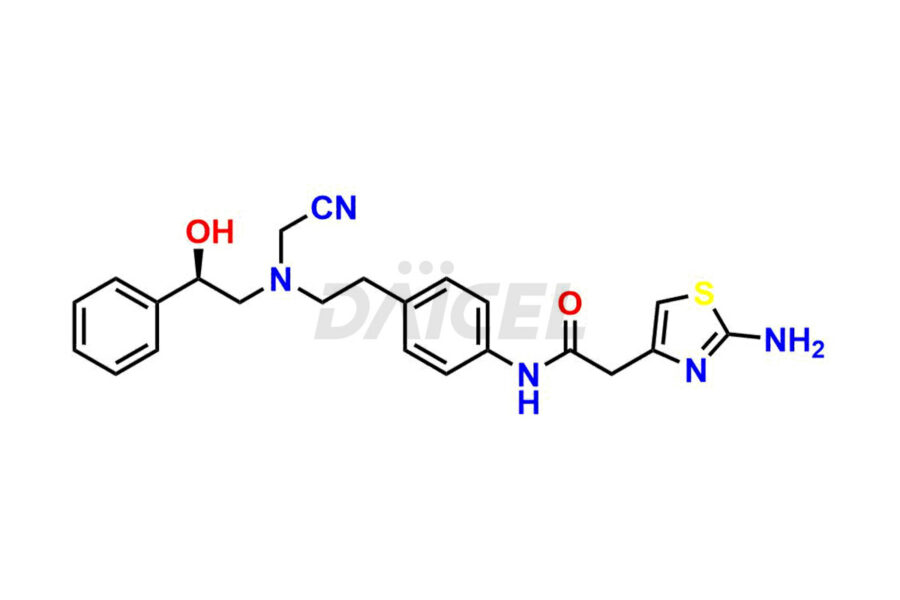
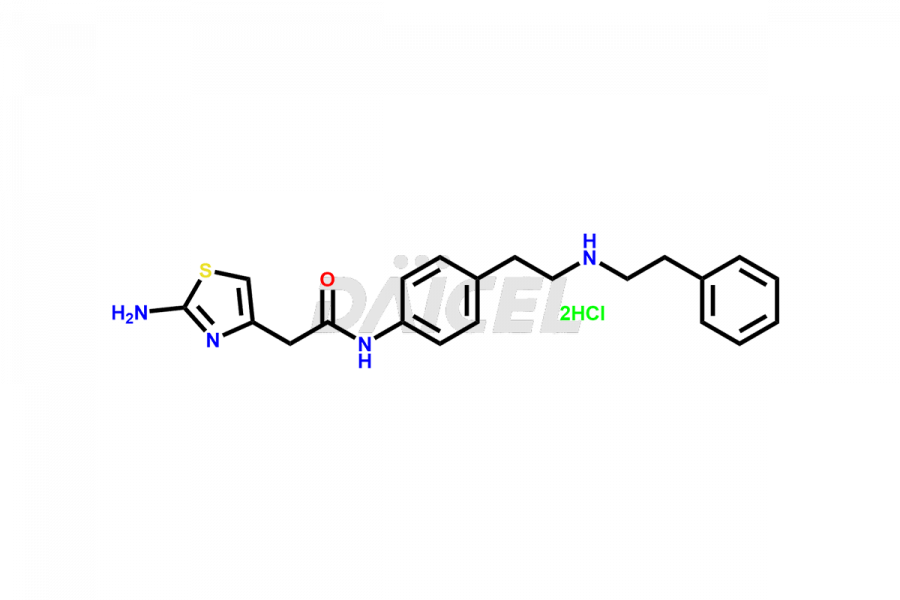
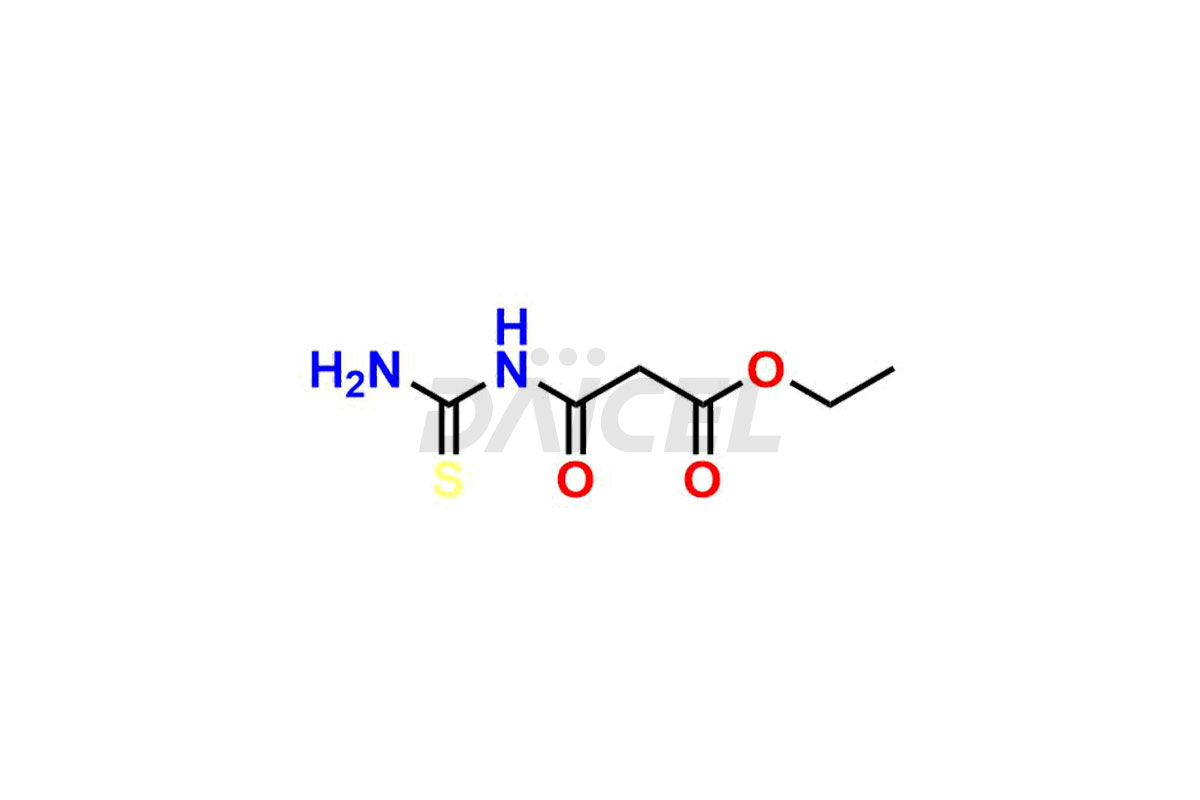
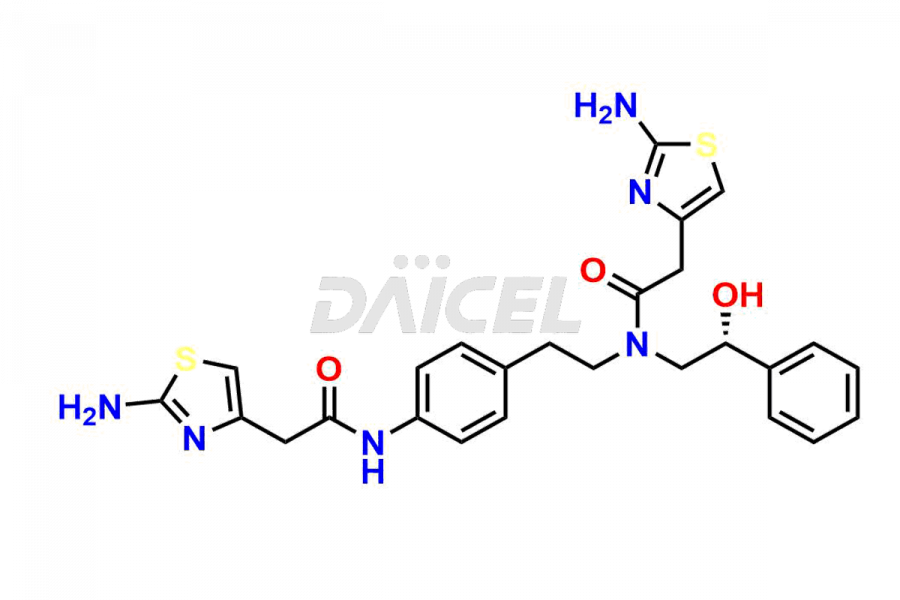
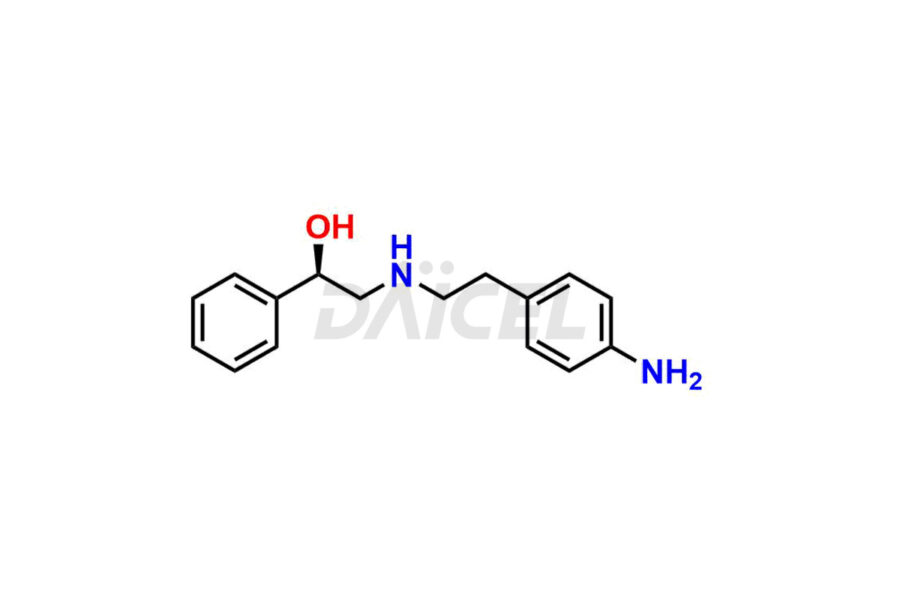
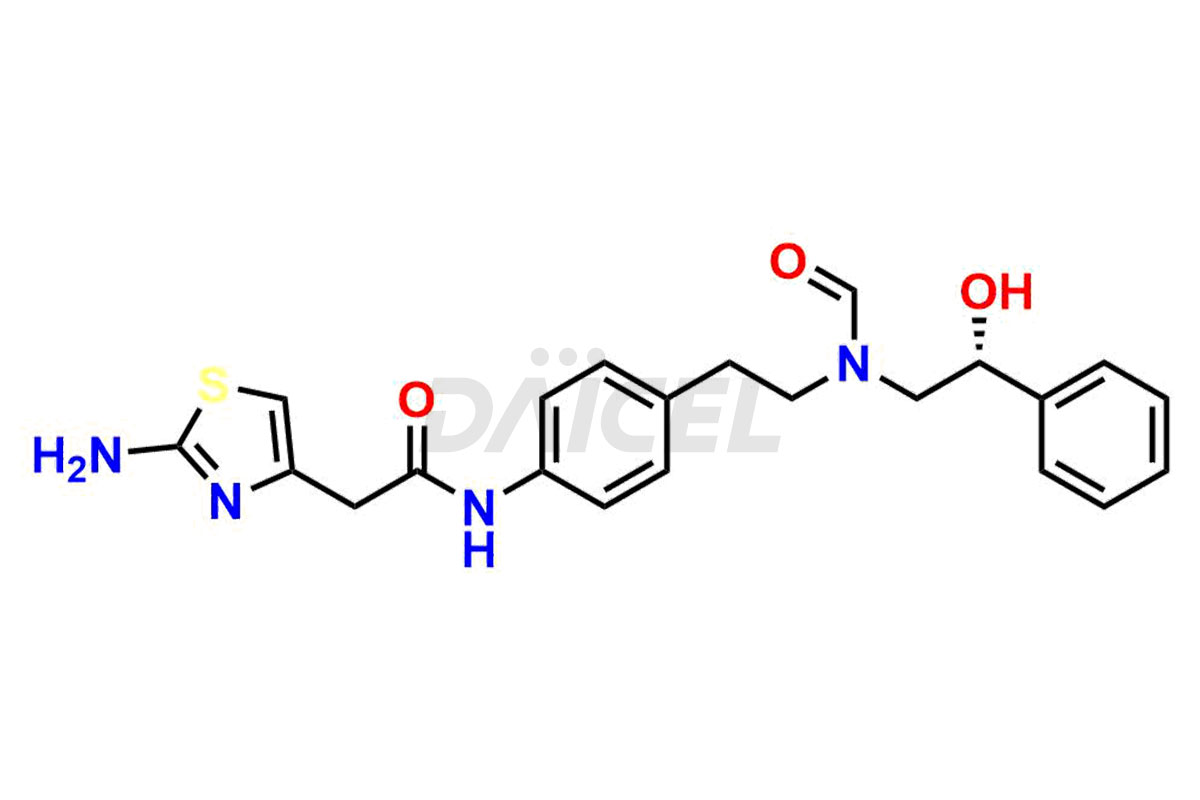
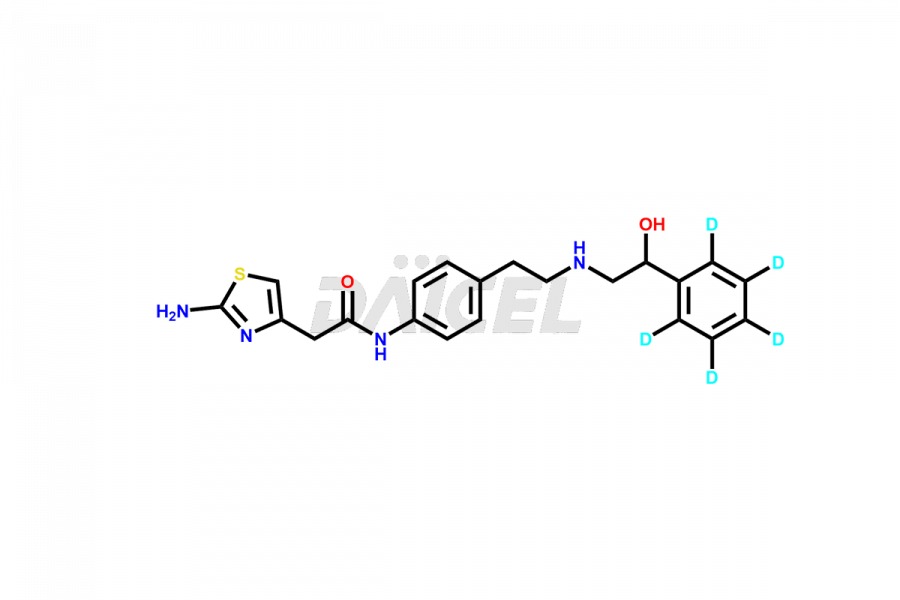
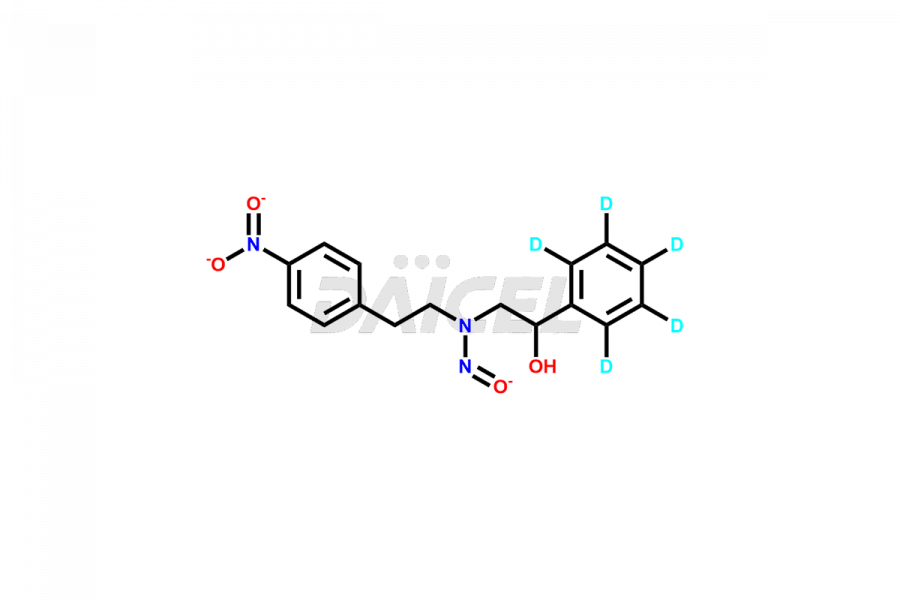
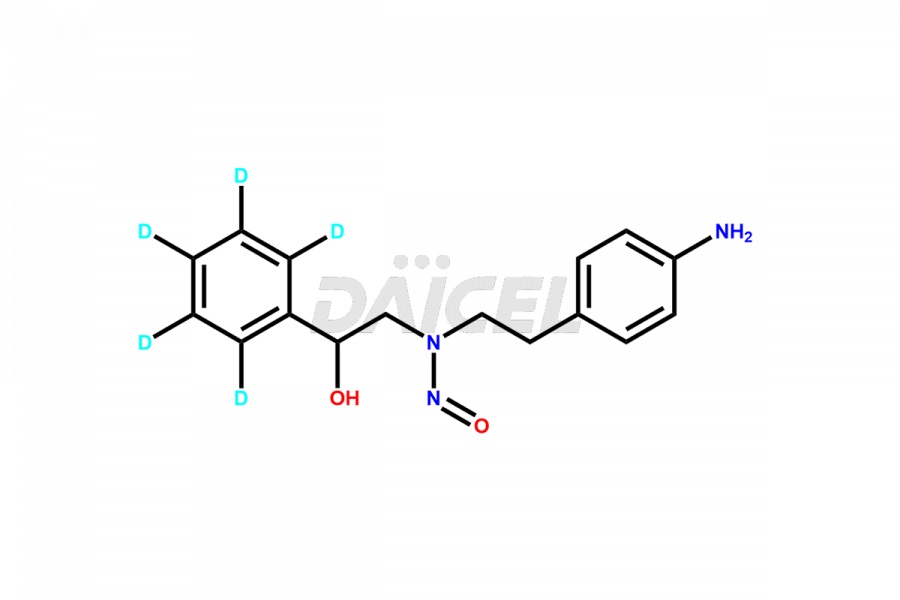
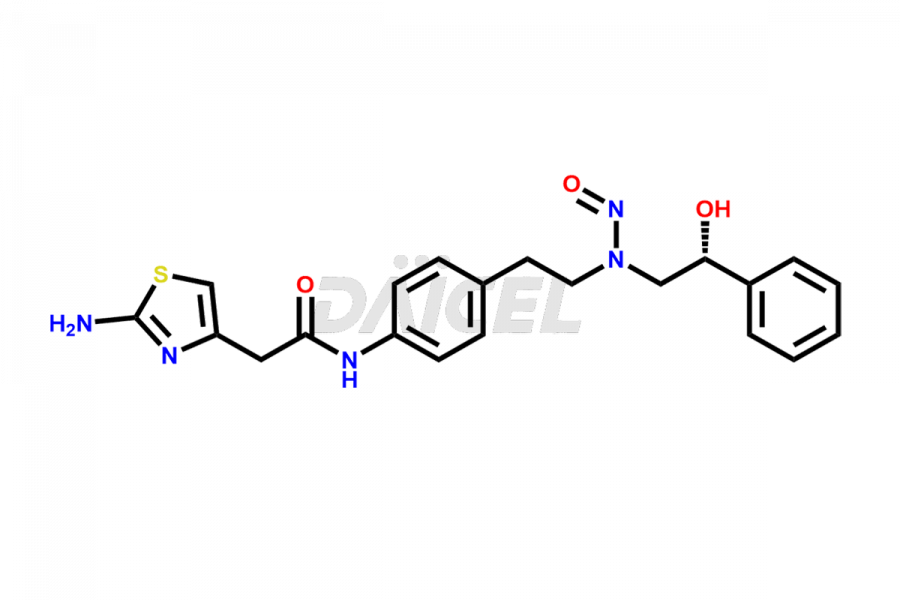
Daicel Pharma is a reliable source for synthesizing high-quality Mirabegron impurities, specifically (S)-Mirabegron, 2-(2-nitrophenyl)ethane amine, 2-(2,3-dinitrophenyl)ethan-1-amine hydrochloride, 2-(2,5-dinitrophenyl)ethan-1-amine hydrochloride, 2-(3-nitrophenyl)ethane amine, 2-(3,4-dinitrophenyl)ethan-1-amine, Cyanomethyl Mirabegron, ethyl 3-oxo-3-thioureidopropanoate, Mirabegron Dimer Impurity, Mirabegron impurity C, Mirabegron Impurity-R, N-Methyl Mirabegron Impurity, N-Nitroso Mirabegron, and N-Nitroso Mirabegron S-Isomer. These impurities help assess the quality, stability, and safety of the active pharmaceutical ingredient, Mirabegron. Daicel Pharma also offers custom synthesis of Mirabegron impurities, which can be shipped worldwide.
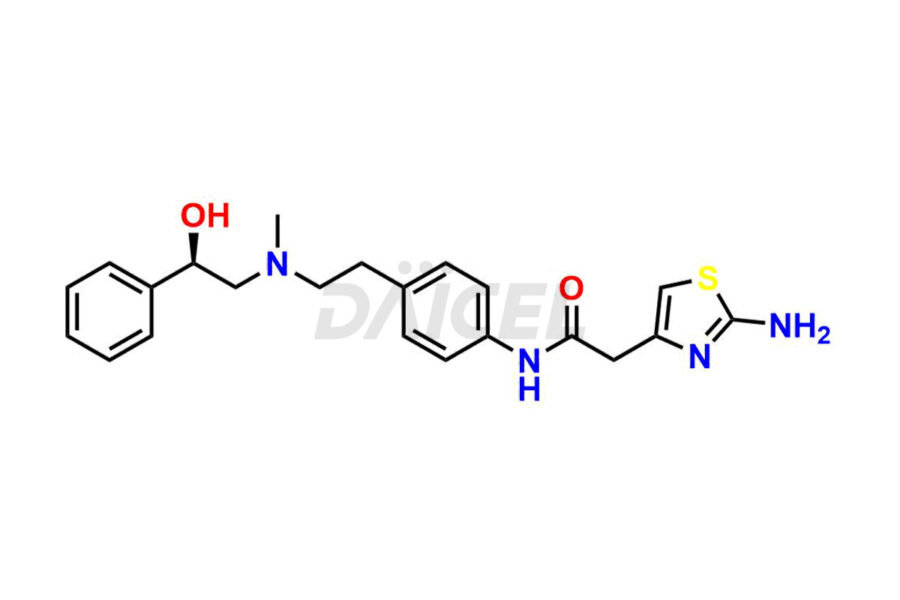
Mirabegron [CAS: 223673-61-8] is an oral medicine that treats an overactive bladder and is an agonist of the human beta-3 adrenergic receptor (ADRB3). It has muscle-relaxing, neuroprotective, and potential antineoplastic effects. Mirabegron is a beta-3 adrenergic agonist that treats overactive bladder syndrome.
Mirabegron
Mirabegron is a medication that treats overactive bladder (OAB), either alone or in combination with solifenacin. It treats neurogenic detrusor overactivity (NDO) in pediatric patients at least three years old and weighing at least 35 kg. Mirabegron is classified as a sympathomimetic medication and is available under the trade name Myrbetriq. The US FDA approved it in 2012 for treating overactive bladder with symptoms of urinary incontinence, urgency, and urinary frequency.
Mirabegron Structure and Mechanism of Action 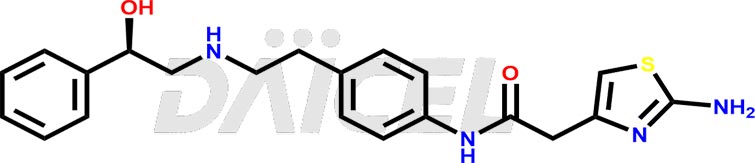
The chemical name of Mirabegron is 2-Amino-N-[4-[2-[[(2R)-2-hydroxy-2-phenylethyl]amino]ethyl]phenyl]-4-thiazoleacetamide. Its chemical formula is C21H24N4O2S, and its molecular weight is approximately 396.5 g/mol.
Mirabegron relaxes the detrusor smooth muscle by activating beta-3 adrenergic receptor (AR) and increases bladder capacity.
Mirabegron Impurities and Synthesis
During the synthesis1 of Mirabegron, impurities form, including the degradation products of Mirabegron, starting materials, and intermediates. These impurities can affect the drug’s quality, safety, and efficacy. Therefore, it is necessary to control and monitor the levels of these impurities during synthesis and after formation of the final product.
Daicel Pharma issues a Certificate of Analysis (CoA) for Mirabegron impurity standards, including (S)-Mirabegron, 2-(2-nitrophenyl)ethane amine, 2-(2,3-dinitrophenyl)ethan-1-amine hydrochloride, 2-(2,5-dinitrophenyl)ethan-1-amine hydrochloride, 2-(3-nitrophenyl)ethane amine, 2-(3,4-dinitrophenyl)ethan-1-amine, Cyanomethyl Mirabegron, ethyl 3-oxo-3-thioureidopropanoate, Mirabegron Dimer Impurity, Mirabegron impurity C, Mirabegron Impurity-R, N-Methyl Mirabegron Impurity, N-Nitroso Mirabegron, and N-Nitroso Mirabegron S-Isomer. The CoA is from an analytical facility that complies with current Good Manufacturing Practices (cGMP) and includes comprehensive characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity. We give additional characterization data such as 13C-DEPT and CHN on request. Daicel Pharma can prepare unknown Mirabegron impurities or degradation products. A complete characterization report accompanies each delivery.
References
FAQ's
References
- Maruyama, Tatsuya; Suzuki, Takayuki; Onda, Kenichi; Hayakawa, Masahiko; Moritomo, Hiroyuki; Kimizuka, Tetsuya; Matsui, Tetsuo, Amide Derivatives Or Salts Thereof, Yamanouchi Pharmaceutical Co., Ltd., Japan, EP1028111B1, May 12, 2004
- van Teijlingen, Raymond; Meijer, John; Takusagawa, Shin; van Gelderen, Marcel; van den Beld, Cas; Usui, Takashi, Development and validation of LC-MS/MS methods for the determination of mirabegron and its metabolites in human plasma and their application to a clinical pharmacokinetic study, Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences, Volume: 887-888, Pages: 102-111,2012
Frequently Asked Questions
Can Mirabegron impurities be removed from the drug product?
Mirabegron impurities removal from the drug product involves various manufacturing processes such as recrystallization, filtration, or chromatography. However, the impurity removal process needs to be validated and effective without affecting the safety and efficacy of the drug product.
Are there any safety concerns related to Mirabegron impurities?
High levels of Mirabegron impurities can harm patients. Therefore, it is necessary to control the impurity levels to ensure drug safety.
Which solvent helps in the analysis of Mirabegron impurities?
Methanol is a solvent used for analyzing many impurities in Mirabegron.
What are the temperature conditions required to store Mirabegron impurities?
Mirabegron impurities are stored at a controlled room temperature between 2-8 ⁰C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.