General Information
Sitagliptin Impurities and Sitagliptin
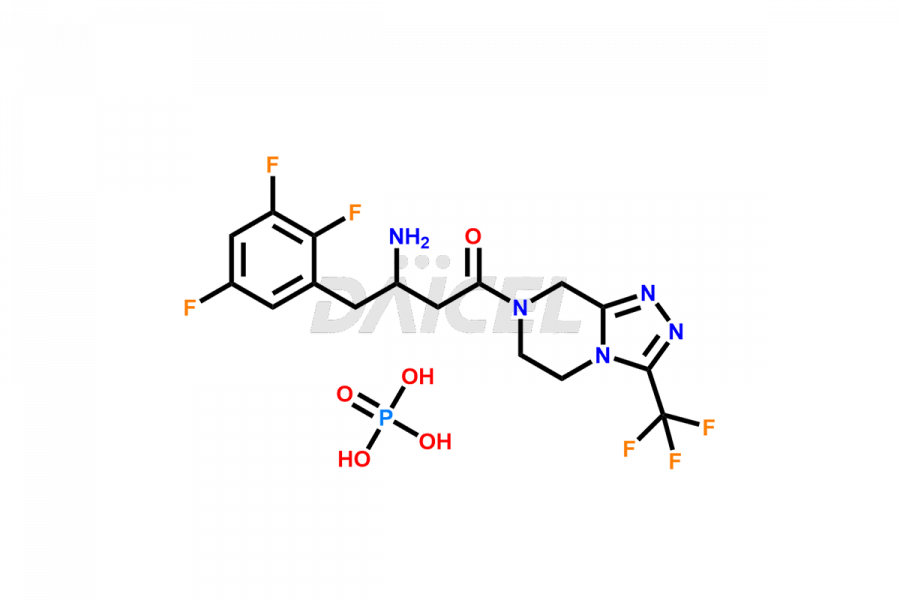
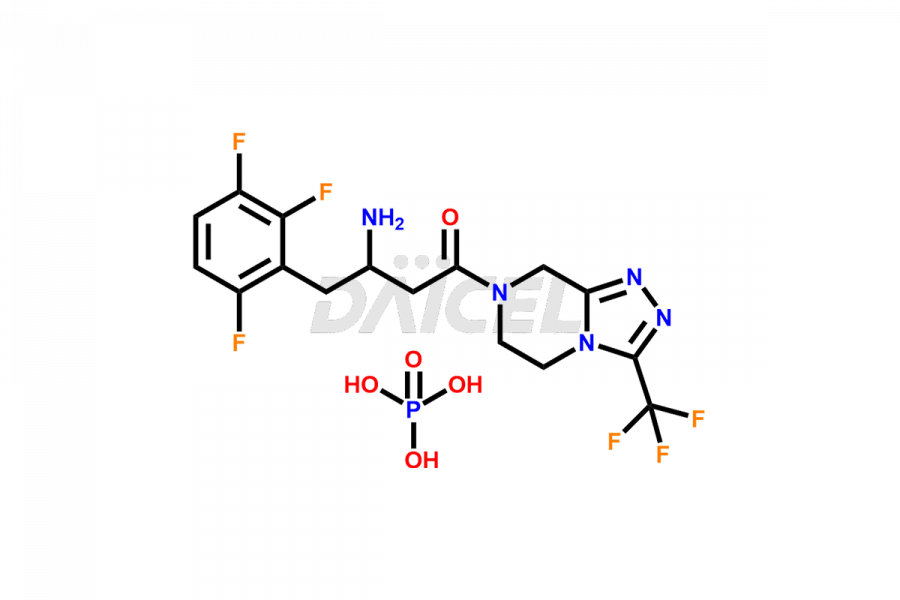
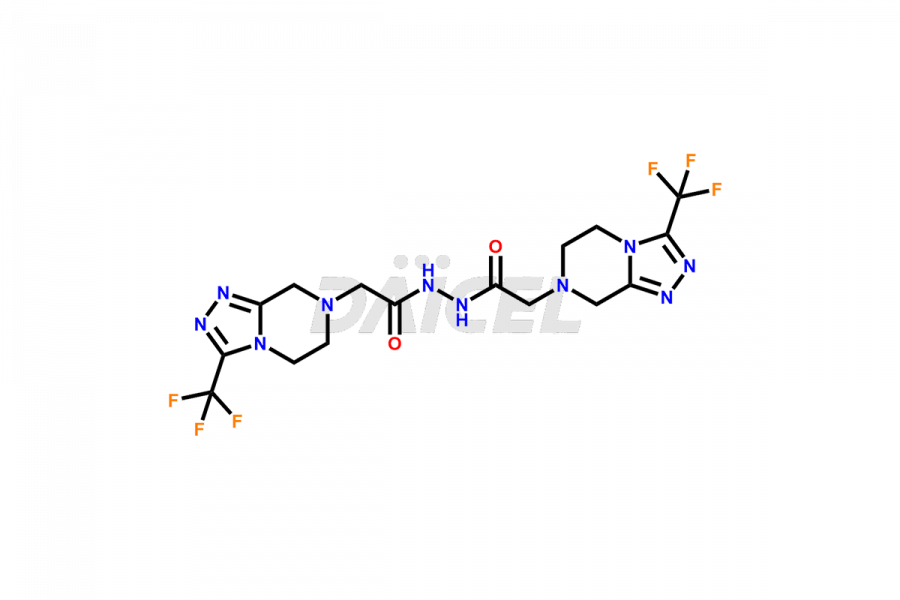
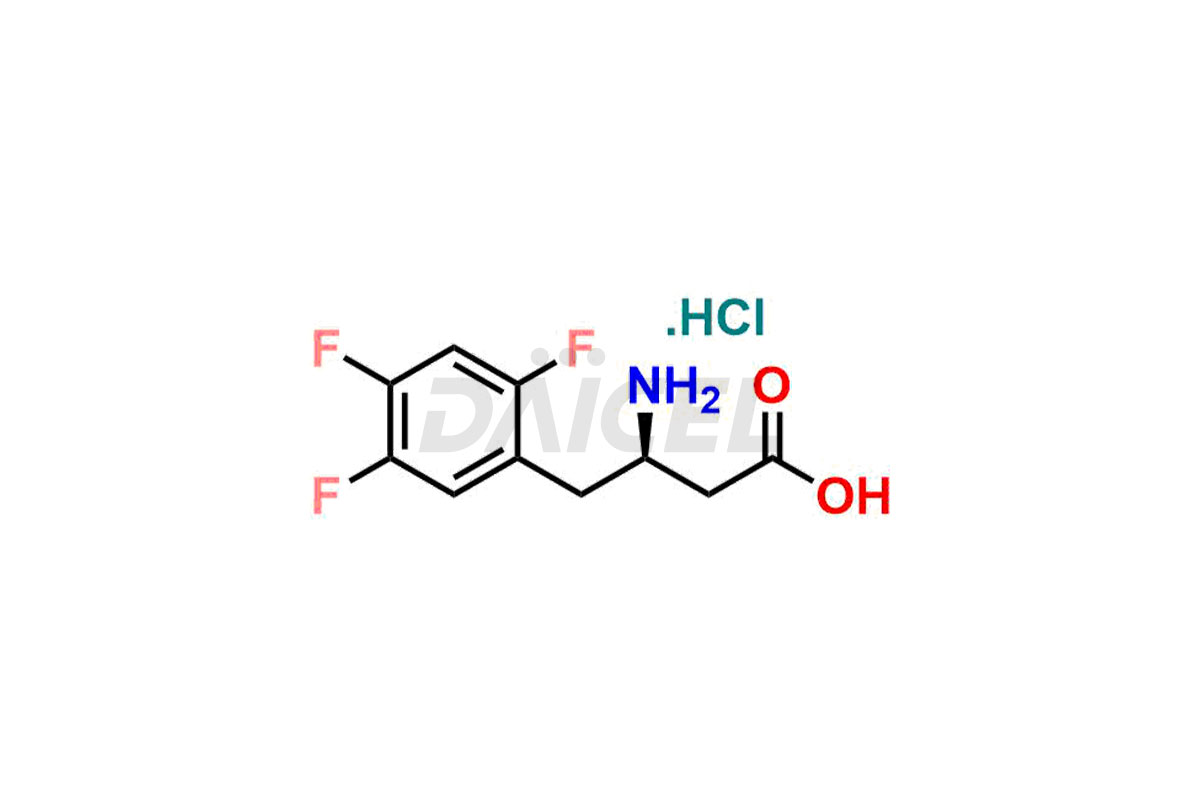
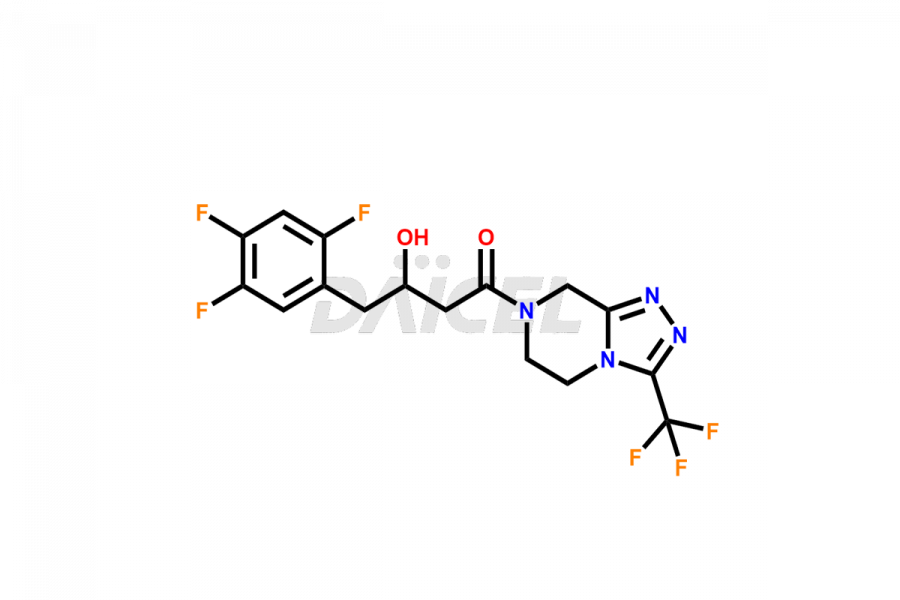
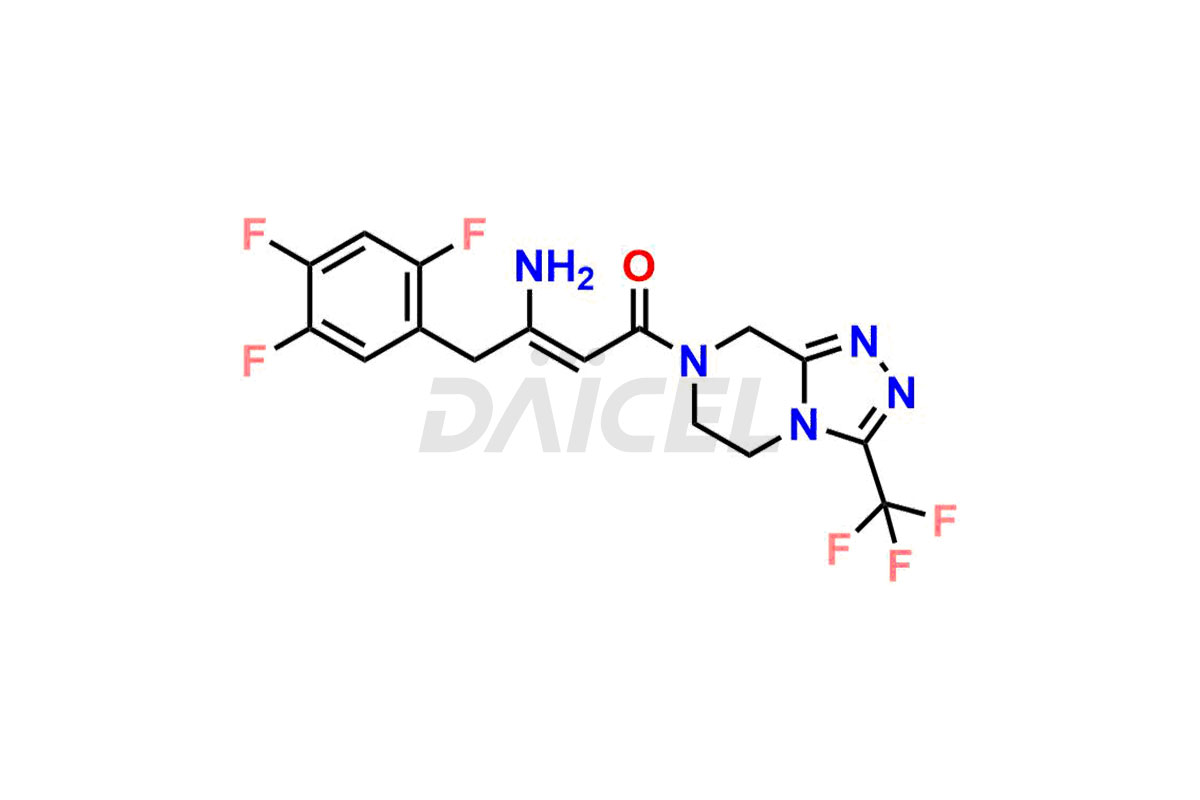
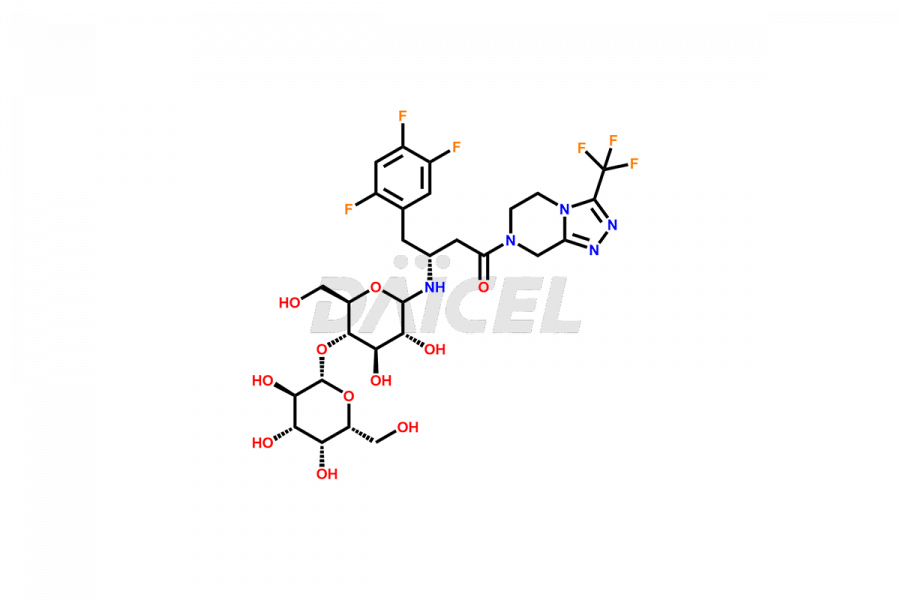
Daicel Pharma synthesizes high-quality Sitagliptin impurities like (S)-Sitagliptin, rac-Sitagliptin, Sitagliptin acid, Sitagliptin alcohol, Sitagliptin Enamine impurity, Sitagliptin hydroxy amide impurity, Sitagliptin Keto Amide Impurity, Sitagliptin Triazecine Analog, and more, which are crucial in the analysis of the quality, stability, and biological safety of the active pharmaceutical ingredient Sitagliptin. Moreover, Daicel Pharma offers custom synthesis of Sitagliptin impurities and delivers them globally.
Sitagliptin [CAS: 486460-32-6] is a hypoglycemic medicine inhibiting the enzyme dipeptidyl peptidase 4 (DDP-4). It treats type 2 diabetes alone or in combination with other oral hypoglycemic agents with diet and exercise.
Sitagliptin: Use and Commercial Availability
Sitagliptin became the first oral DPP-4 inhibitor in 2006 to be approved by the FDA for treating Type 2 Diabetes. It is effective in reducing HbA1c levels and fasting glucose levels while improving postprandial glucose excursions. It is approved as a monotherapy and an add-on therapy to metformin or glitazone when metformin plus diet is not enough. Sitagliptin’s main effects are on islet cell function. Janumet, Januvia, and Steglujan are the brands under which Sitagliptin is available.
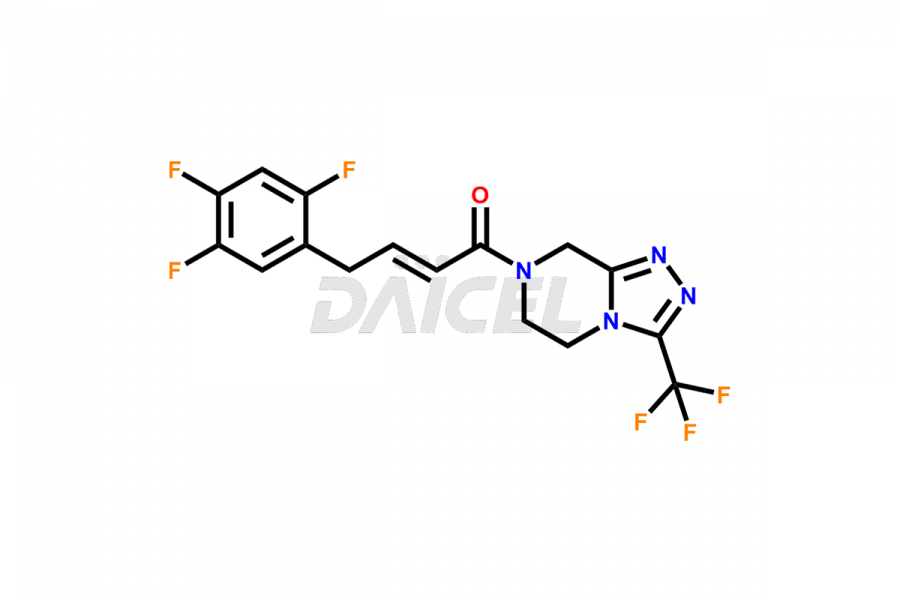
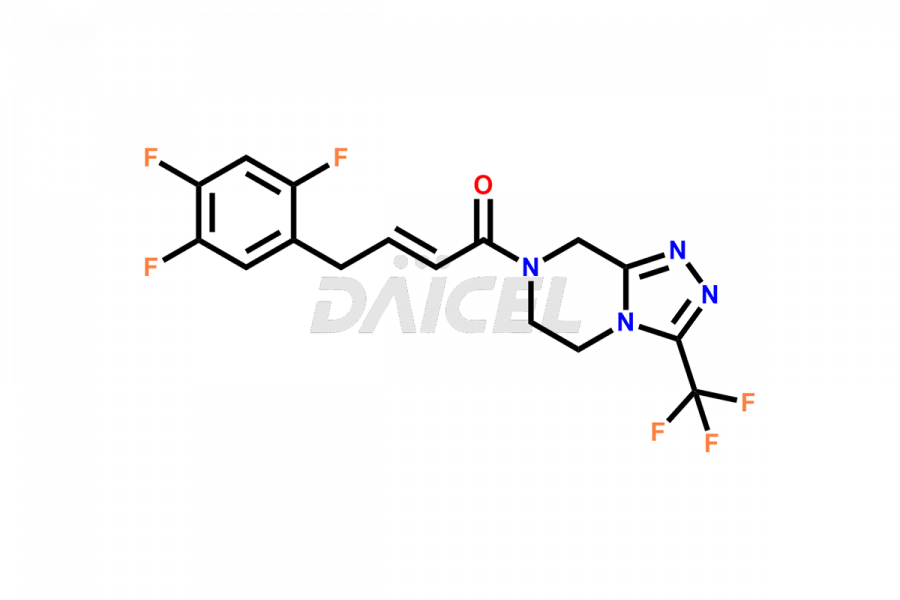
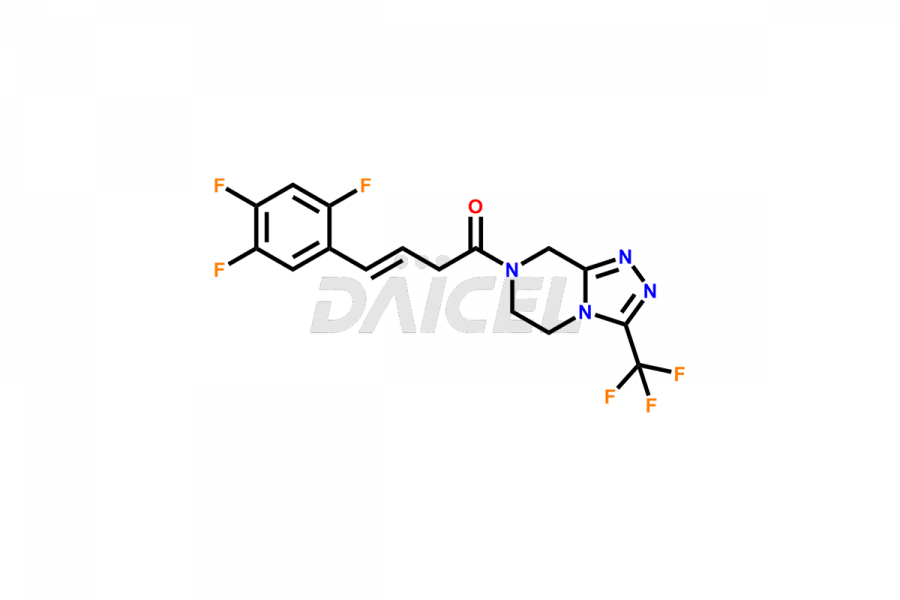
Sitagliptin Structure and Mechanism of Action 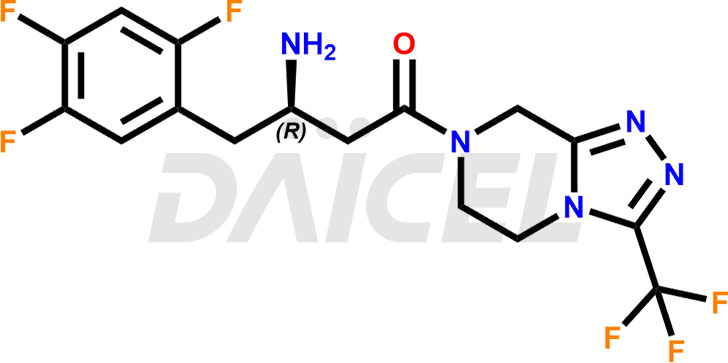
The chemical name of Sitagliptin is (3R)-3-Amino-1-[5,6-dihydro-3-(trifluoromethyl)-1,2,4-triazolo[4,3-a]pyrazin-7(8H)-yl]-4-(2,4,5-trifluorophenyl)-1-butanone. Its chemical formula is C16H15F6N5O, and its molecular weight is approximately 407.31 g/mol.
Sitagliptin, a DPP-4 inhibitor, treats patients with type-2 diabetes by slowing the inactivation of incretin hormones. Incretin hormones help in the physiologic regulation of glucose hemostasis.
Sitagliptin Impurities and Synthesis
Sitagliptin may form impurities during its manufacturing1. These impurities may harm patients influenced by factors, reaction conditions, starting materials, and purification methods. Strict quality control measures must be in place to monitor and remove impurities during manufacturing to ensure the purity and safety of the final product.
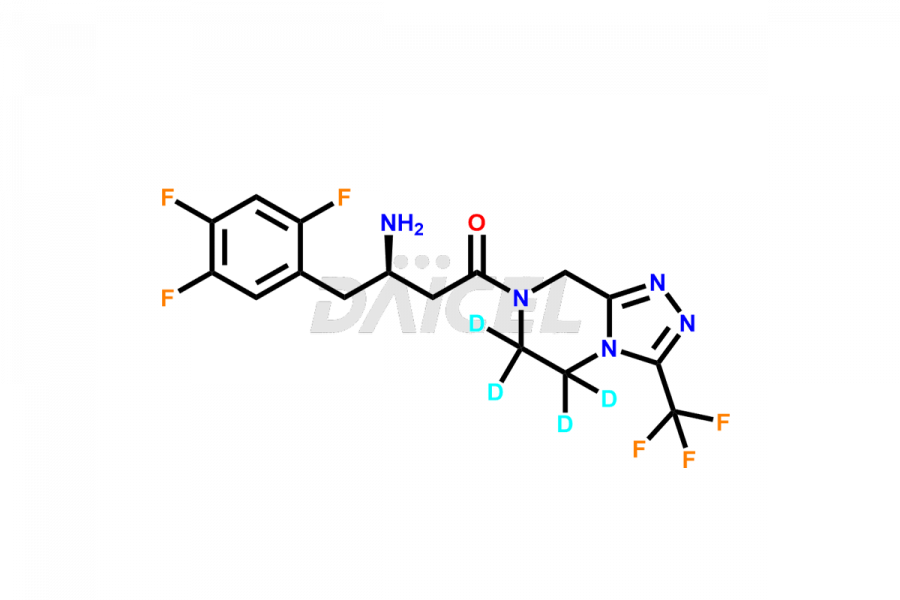
Daicel provides a Certificate of Analysis (CoA) for Sitagliptin impurity standards, including (S)-Sitagliptin, rac-Sitagliptin, Sitagliptin acid, Sitagliptin alcohol, Sitagliptin Enamine impurity, Sitagliptin hydroxy amide impurity, Sitagliptin Keto Amide Impurity, Sitagliptin Triazecine Analog, and more. The CoA is issued from a cGMP-compliant analytical facility and contains complete characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. Additional characterization data, such as 13C-DEPT and CHN, can be provided upon request. Daicel can also prepare any unknown Sitagliptin impurity or degradation product and offer labeled compounds to quantify the efficacy of generic Sitagliptin. Daicel offers Sitagliptin Nitroso Impurity-D4 and Sitagliptin D4, deuterium-labeled Sitagliptin compounds used in bio-analytical research such as BA/BE studies. We give a complete characterization report on delivery.