General Information
Sildenafil Impurities and Sildenafil
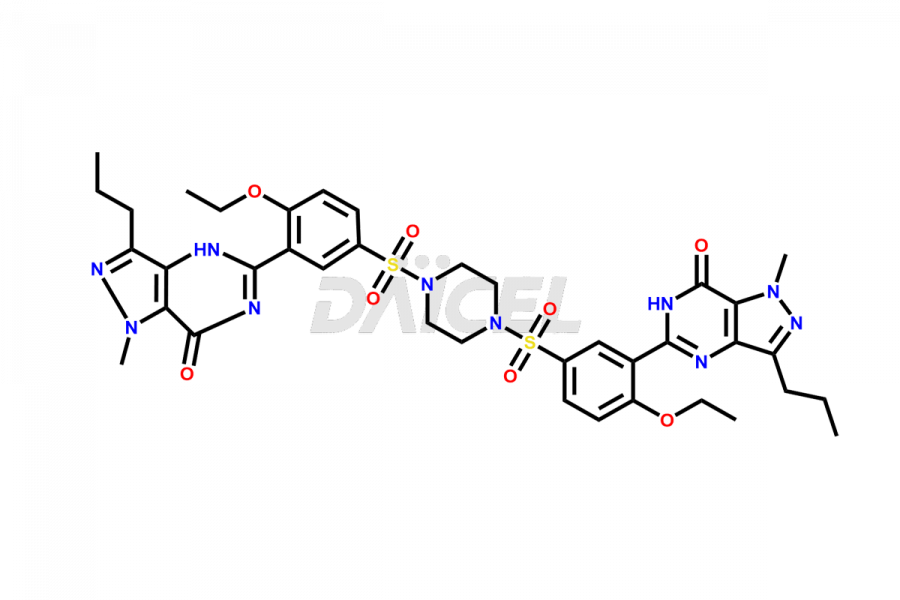
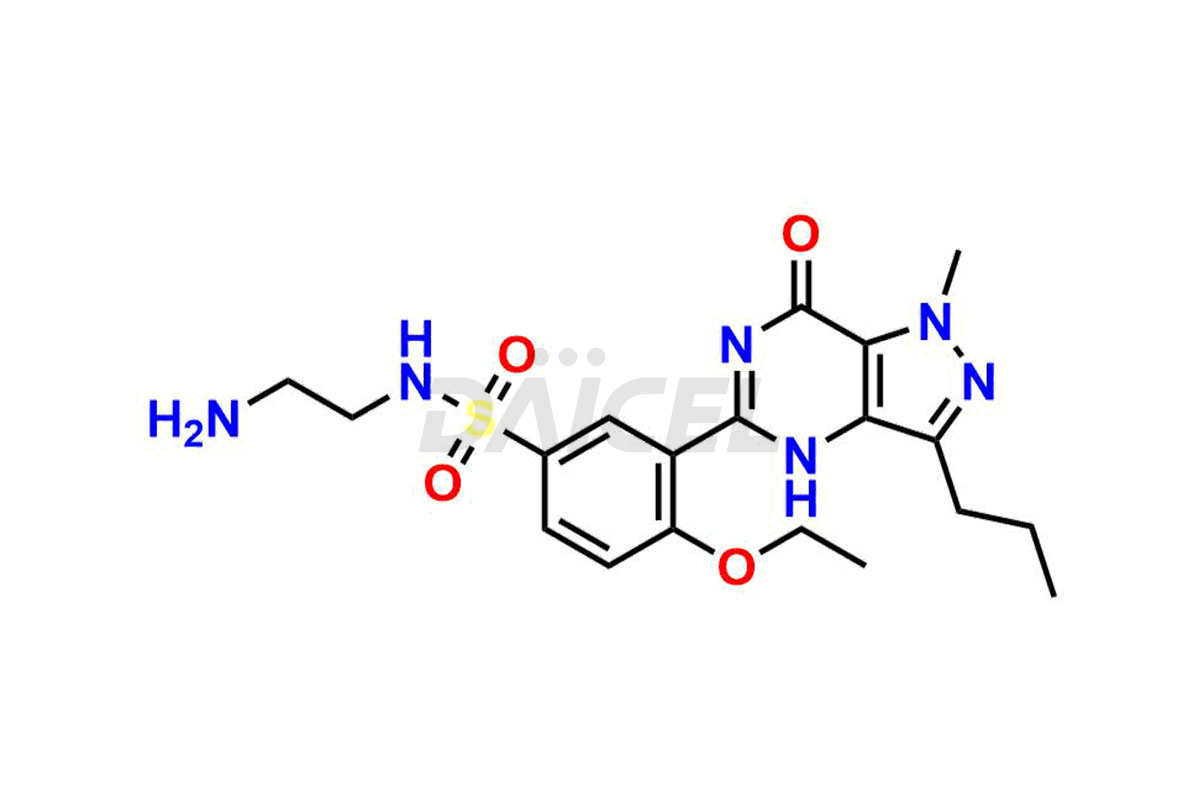
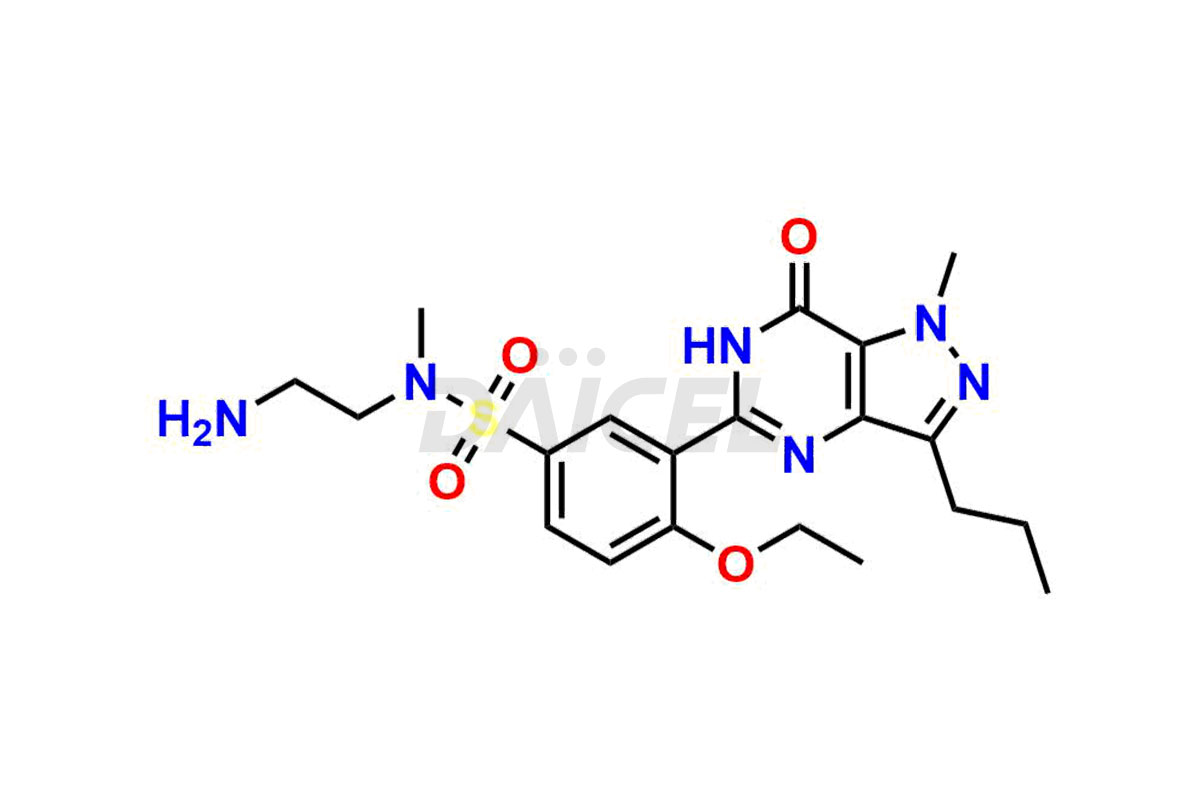
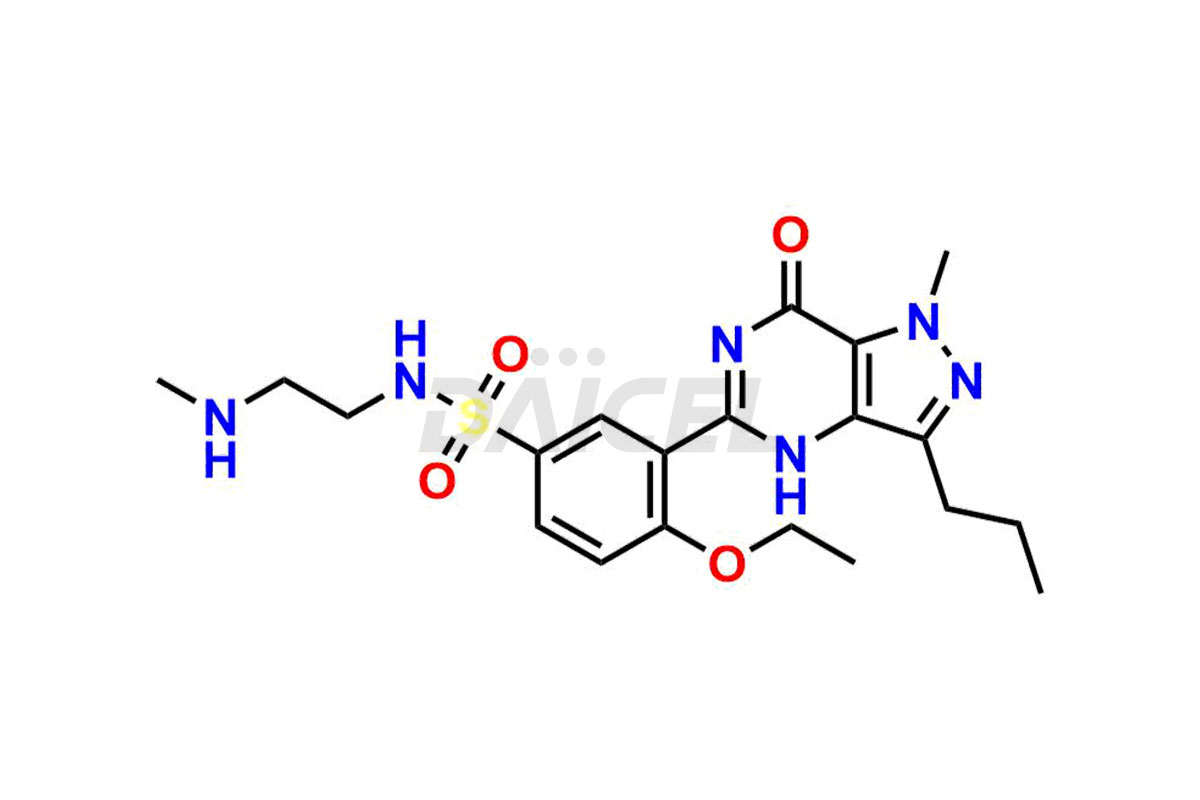
Daicel Pharma is a reliable source for synthesizing high-quality Sildenafil impurities, like Descarbon Sildenafil, Homo Sildenafil, N-desmethyl N-Benzyl Sildenafil, Sildenafil EP Impurity D, and many more. Daicel Pharma offers a range of Sildenafil impurities that help assess active pharmaceutical ingredients’ quality, stability, and safety. Additionally, Daicel Pharma specializes in custom synthesis of Sildenafil impurities, fulfilling specific client requirements. These high-quality impurities can be shipped worldwide, providing flexibility and convenience to customers.
Sildenafil [CAS: 139755-83-2] is a reversible phosphodiesterase-5 (PDE5) inhibitor that treats erectile dysfunction and pulmonary hypertension. The US FDA approves Sildenafil for treating pulmonary arterial hypertension (PAH) in adults belonging to WHO Group I.
Sildenafil: Use and Commercial Availability
Sildenafil is a widely used medication with multiple applications. It is primarily known for its effectiveness in treating erectile dysfunction (ED), a condition characterized by the inability to achieve or maintain an erection sufficient for sexual activity. Sildenafil works by inhibiting the enzyme phosphodiesterase-5 (PDE5), which promotes increased blood flow to the penis, facilitating an erection.
This drug is also crucial in treating pulmonary arterial hypertension (PAH). PAH is a type of high blood pressure that affects the arteries in the lungs and the right side of the heart. Sildenafil helps relax and dilate the blood vessels in the lungs, reducing heart workload and improving exercise capacity in patients with PAH.
Sildenafil is available under the brand names Liqrev, Revatio, and Viagra, which adequately contains the active ingredient, Sildenafil.
Sildenafil Structure and Mechanism of Action 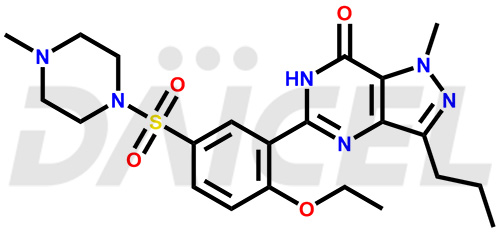
The chemical name of Sildenafil is 5-[2-Ethoxy-5-[(4-methyl-1-piperazinyl)sulfonyl]phenyl]-1,6-dihydro-1-methyl-3-propyl-7H-pyrazolo[4,3-d]pyrimidin-7-one. Its chemical formula is C22H30N6O4S, and its molecular weight is approximately 474.6 g/mol.
Sildenafil enhances the effect of nitric oxide (NO) in the corpus cavernosum. It inhibits phosphodiesterase type 5 (PDE5), which causes the radiation of cGMP in the corpus cavernosum. Sildenafil increases cyclic guanosine monophosphate (cGMP) in the corpus cavernosum leading to smooth muscle relaxation and the inflow of blood to the corpus cavernosum.
Sildenafil Impurities and Synthesis
During the manufacturing1 of Sildenafil, the formation of impurities is possible, which can compromise its effectiveness. These impurities can arise from various sources, including the raw materials, intermediates, and chemicals utilized to synthesize Sildenafil. Close management and monitoring of impurities are paramount to ensure the drug’s optimal efficacy and safety.
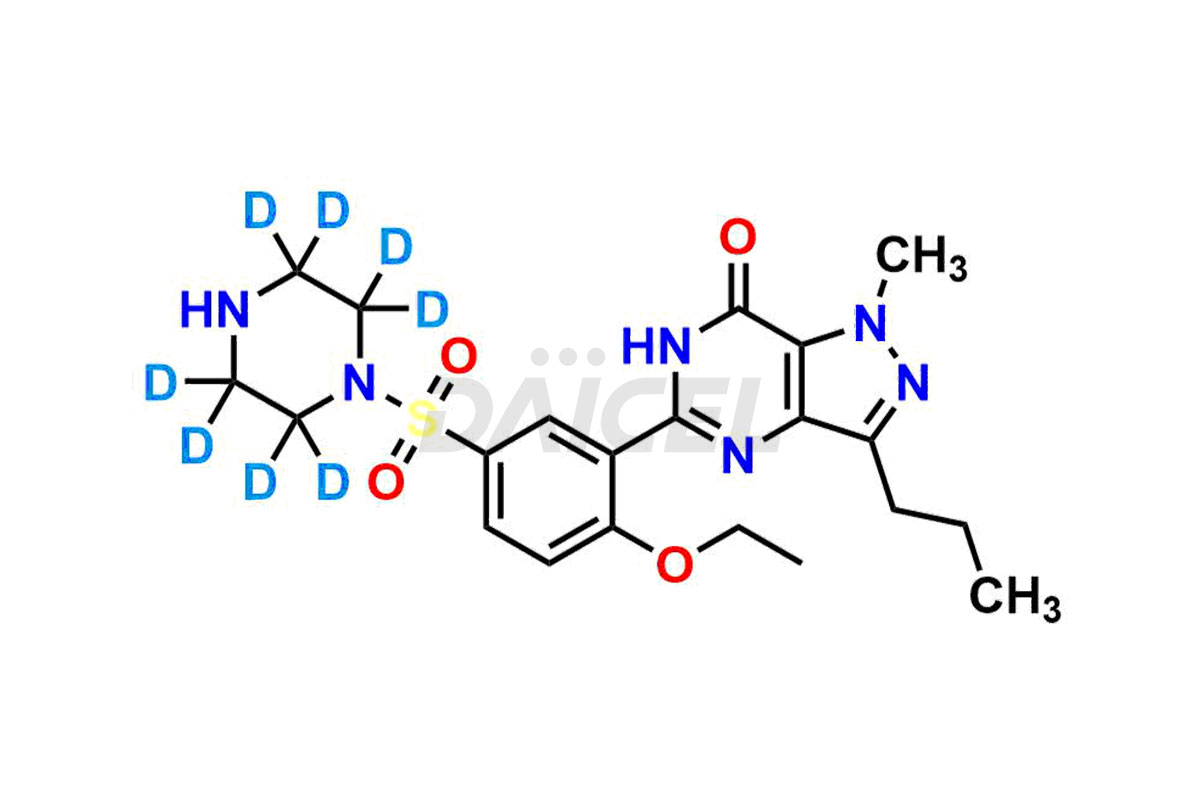
Daicel Pharma offers a wholesome and integrated Certificate of Analysis (CoA) for Sildenafil impurity standards, encompassing impurities such as Descarbon Sildenafil, Homo Sildenafil, N-desmethyl N-Benzyl Sildenafil, Sildenafil EP Impurity D, and many others. The CoA provides detailed characterization data, including 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. Additionally, we give a complete 13C-DEPT on delivery. With advanced technology and expertise, we can synthesize any unknown Sildenafil impurity or degradation product. Daicel Pharma also supplies labeled compounds, facilitating the quantification of generic Sildenafil’s efficacy. For bioanalytical research and BA/BE studies, Daicel Pharma offers Sildenafil– D3, Sildenafil-D8, and Piperazine N-desmethyl Sildenafil D8, deuterium-labeled standards of Sildenafil.