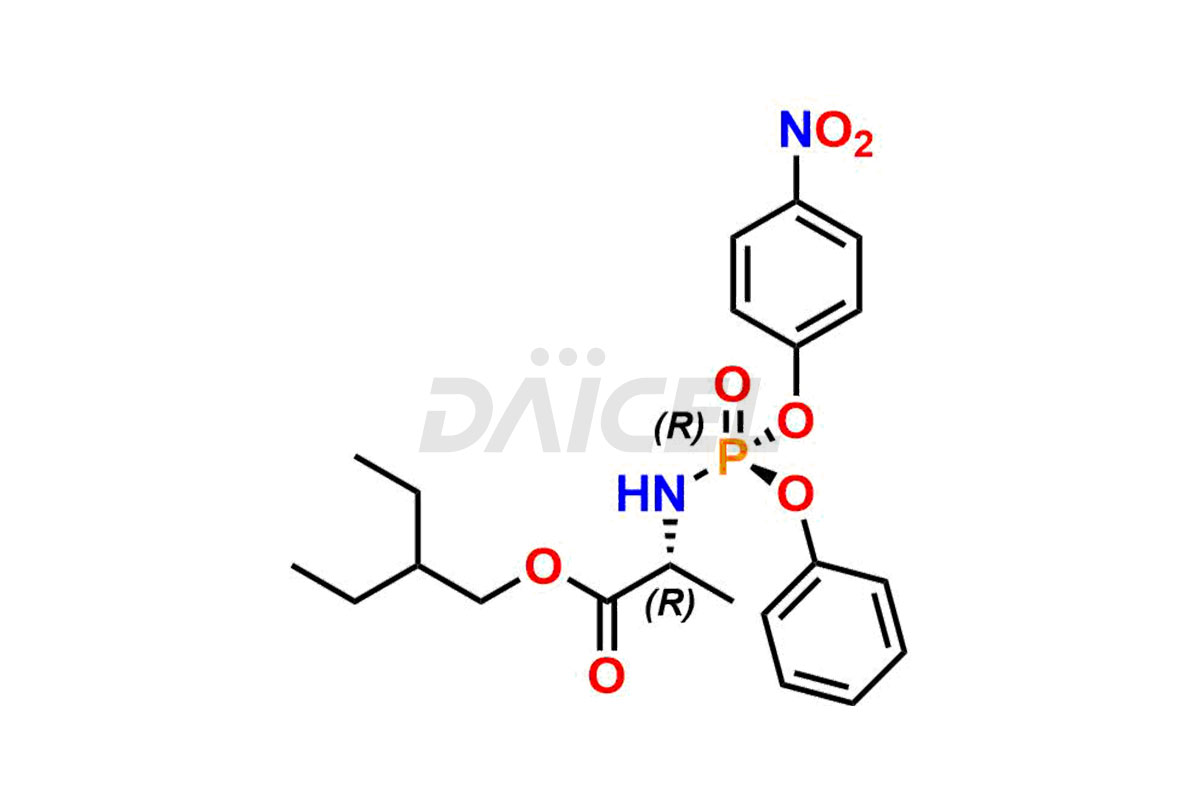
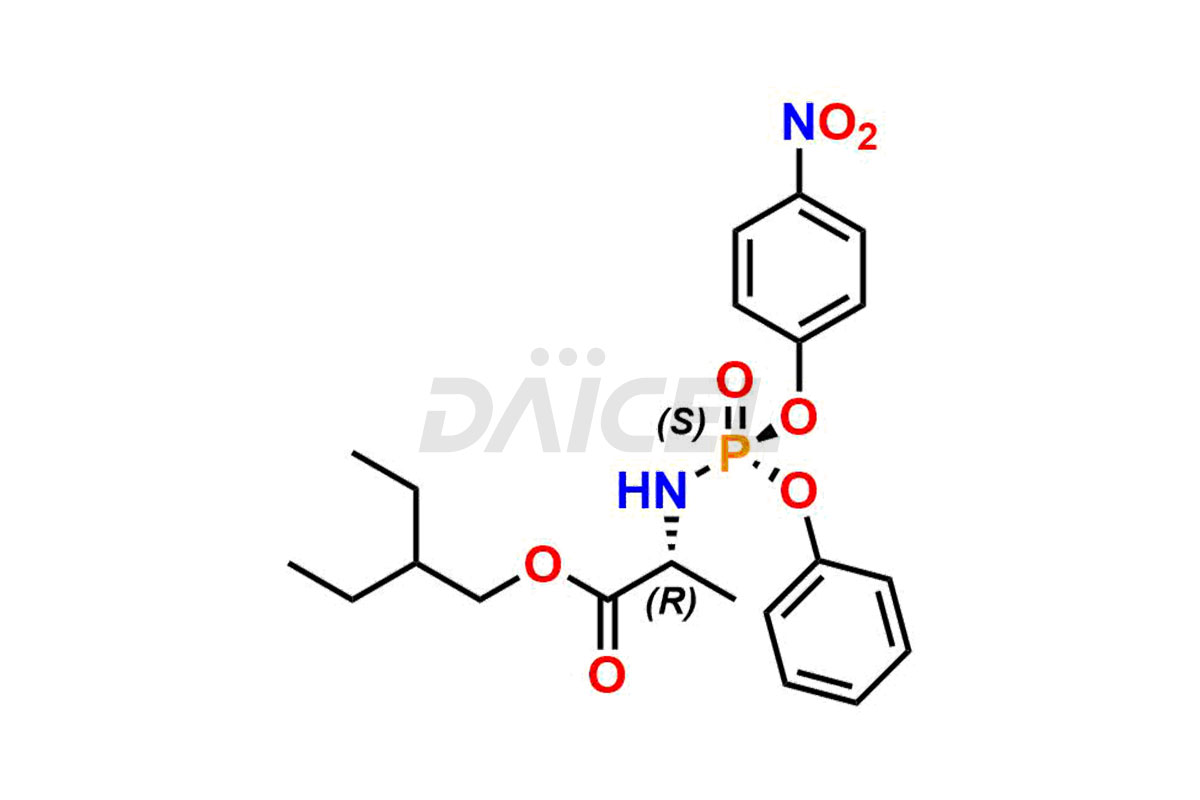
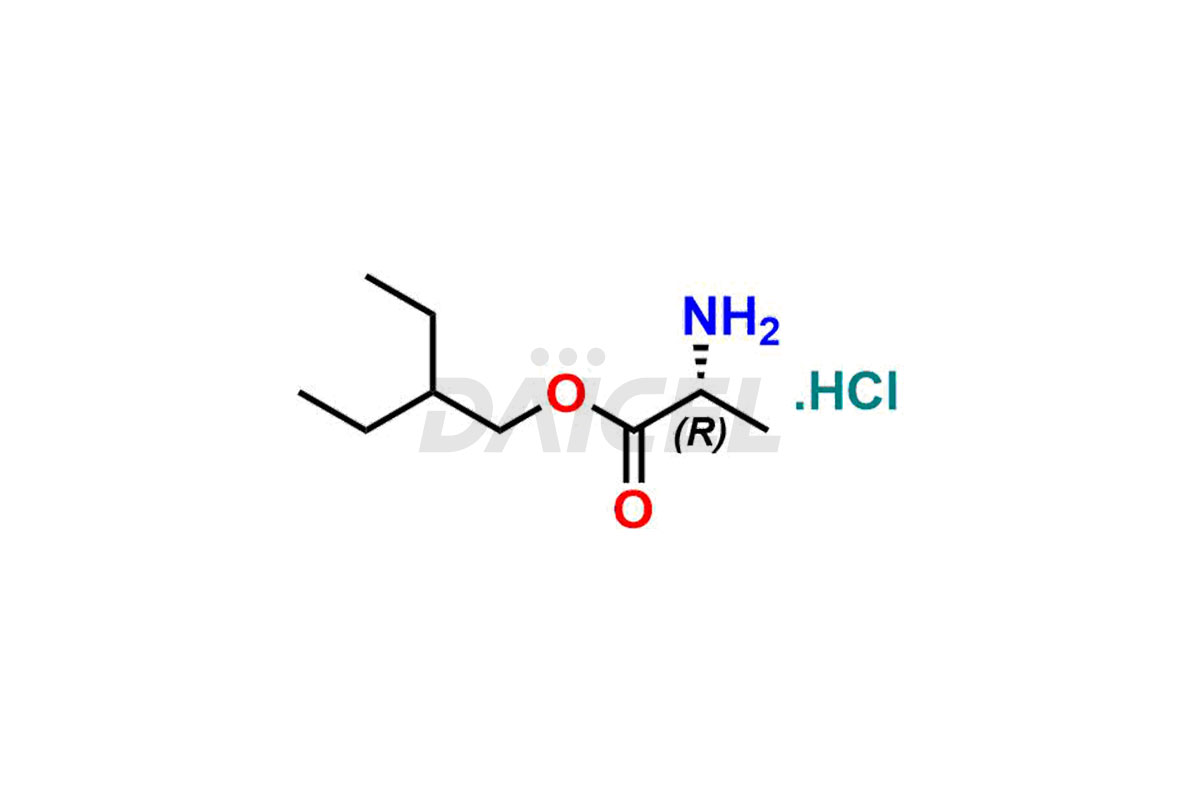
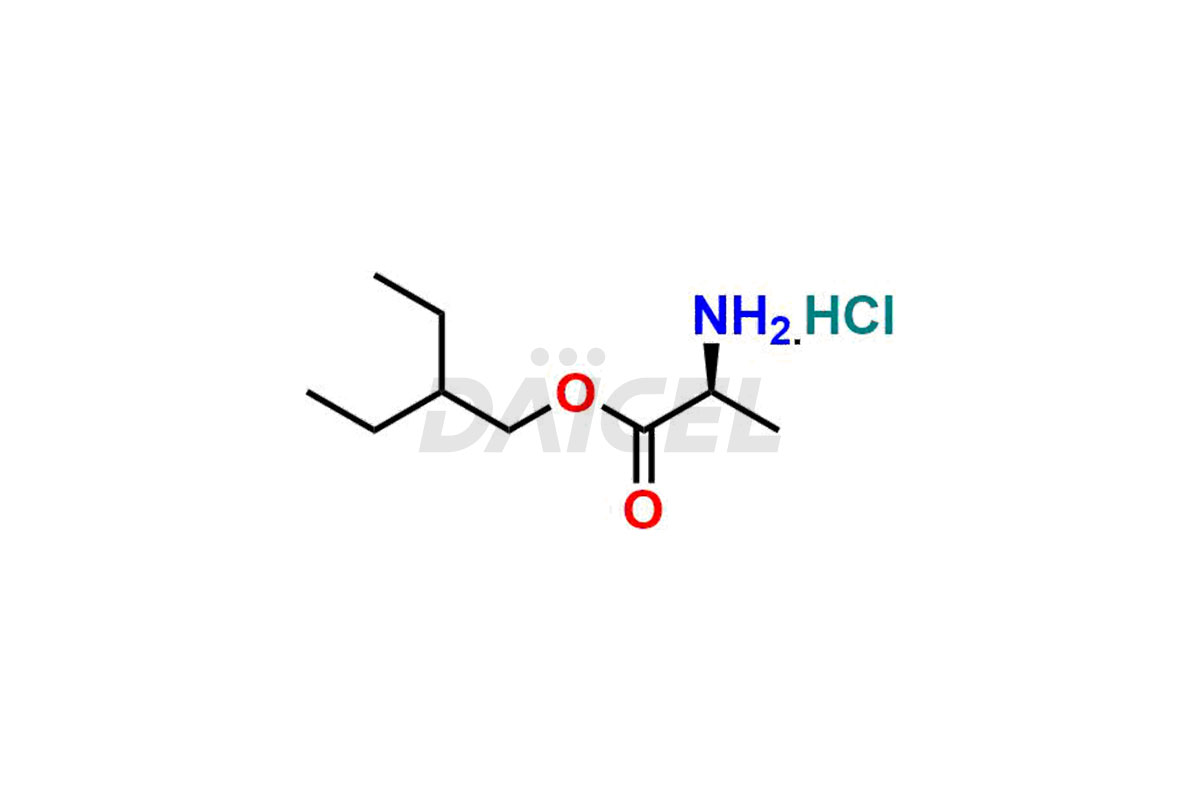
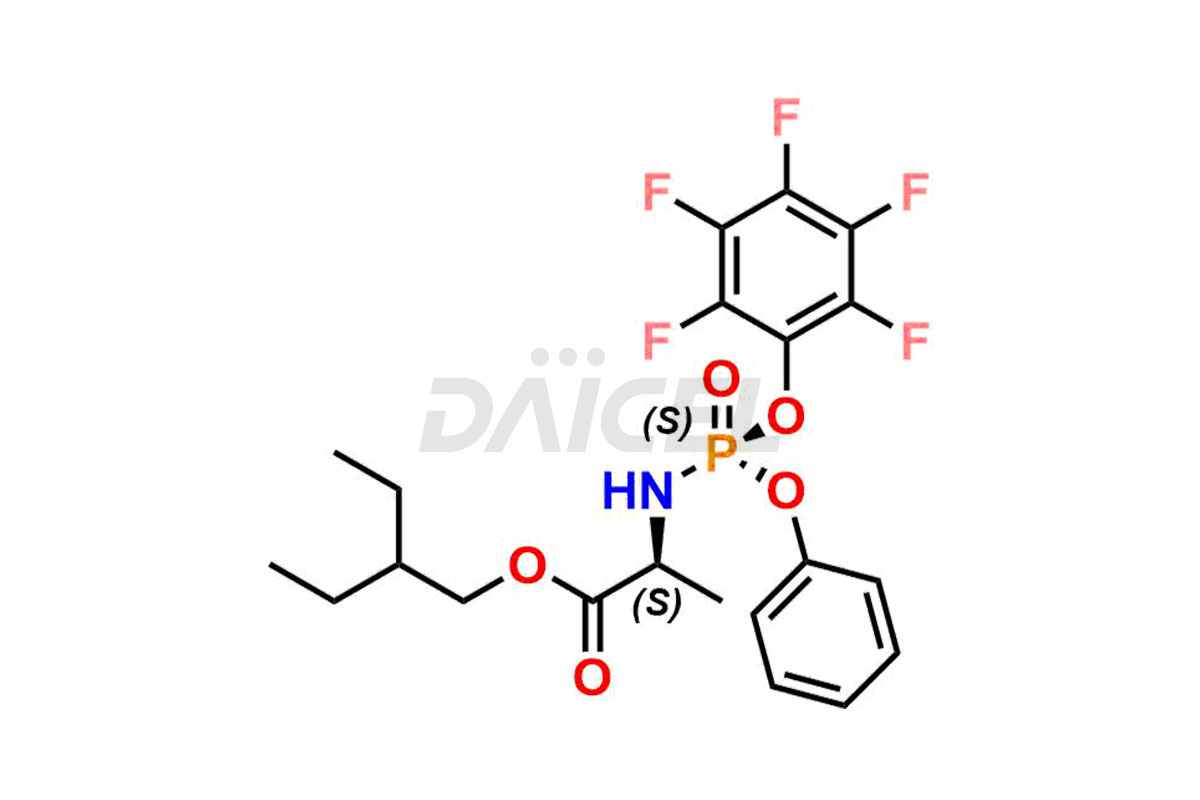
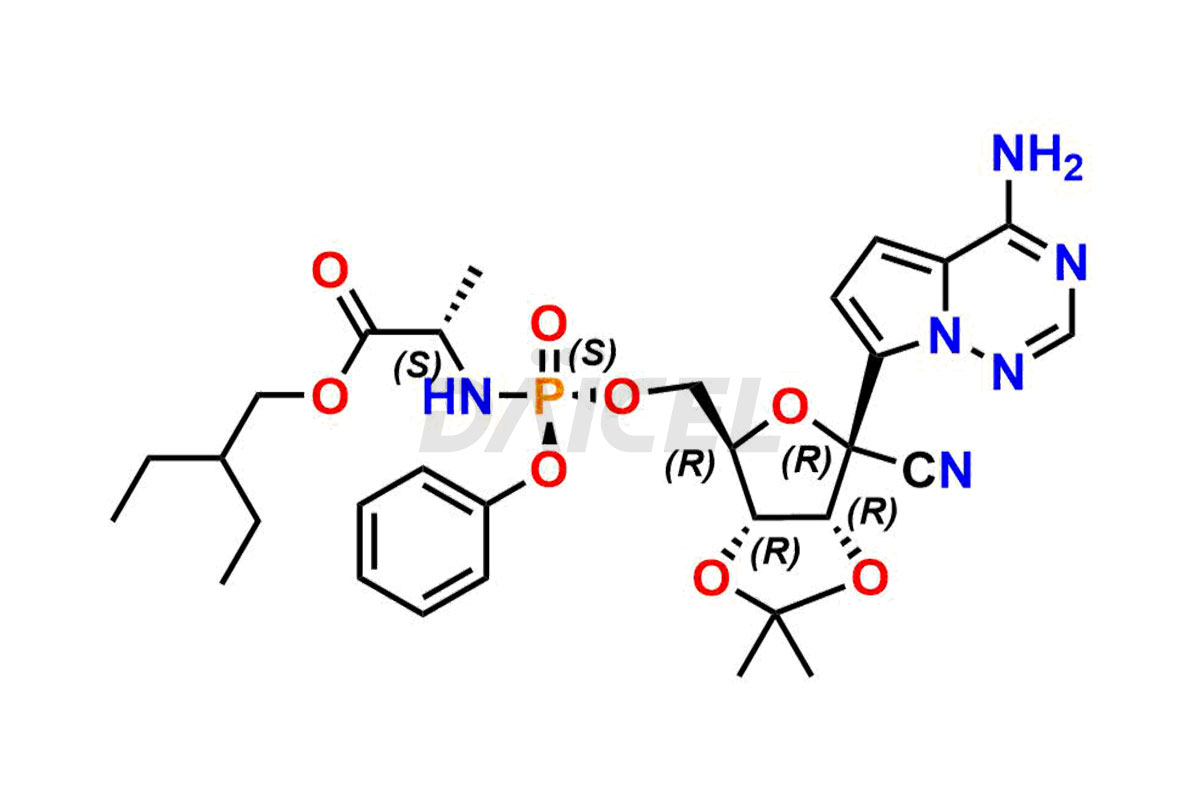
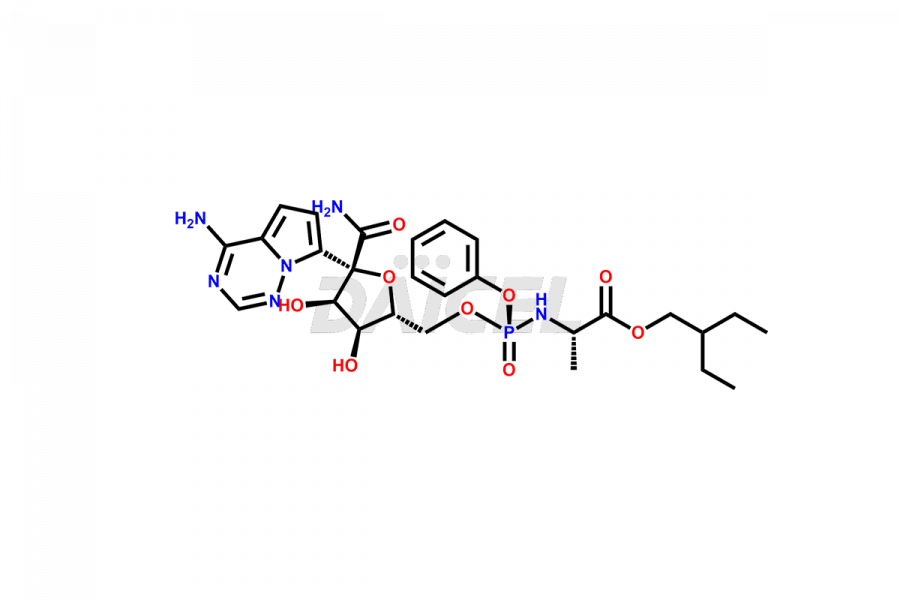
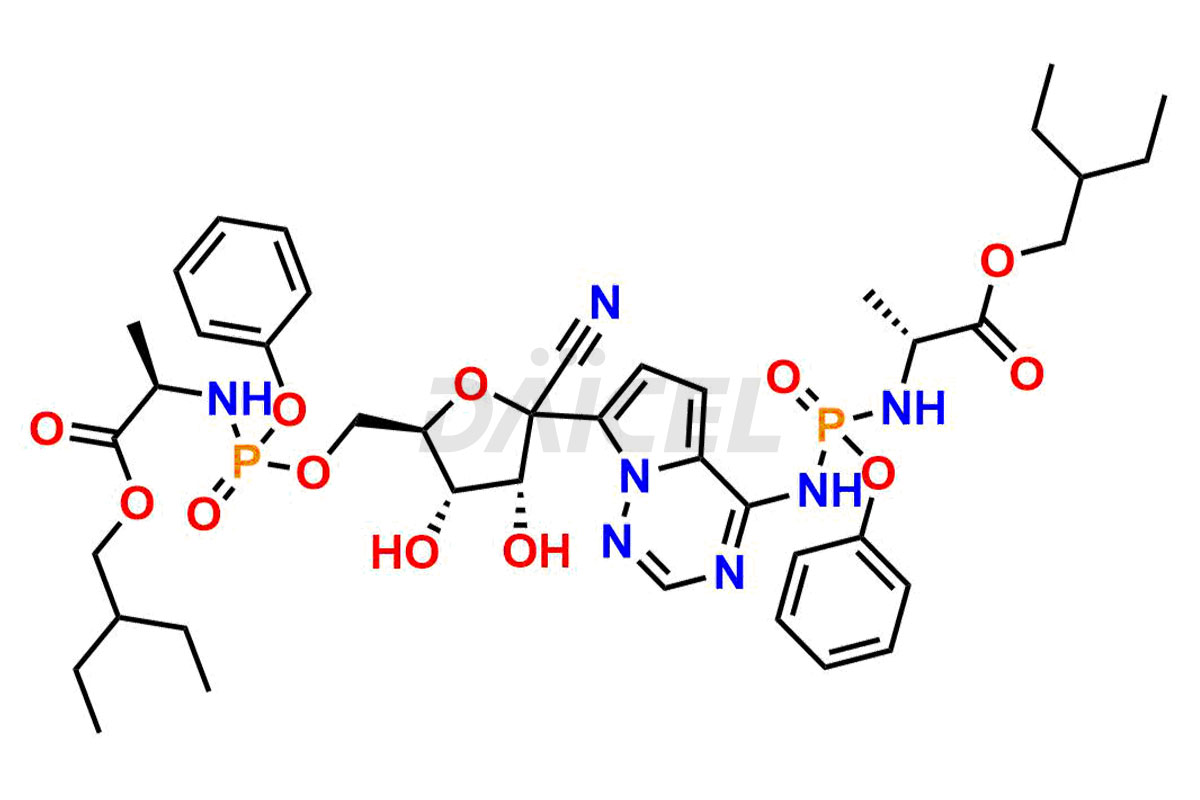
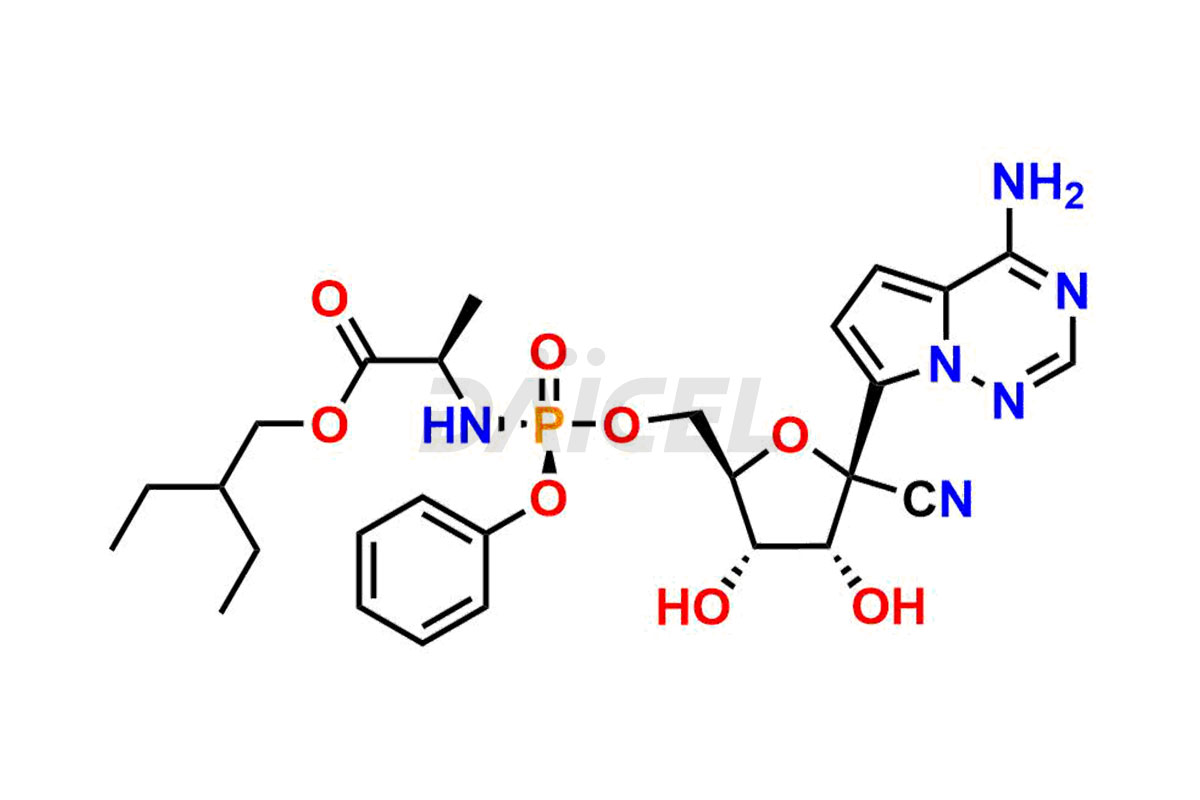
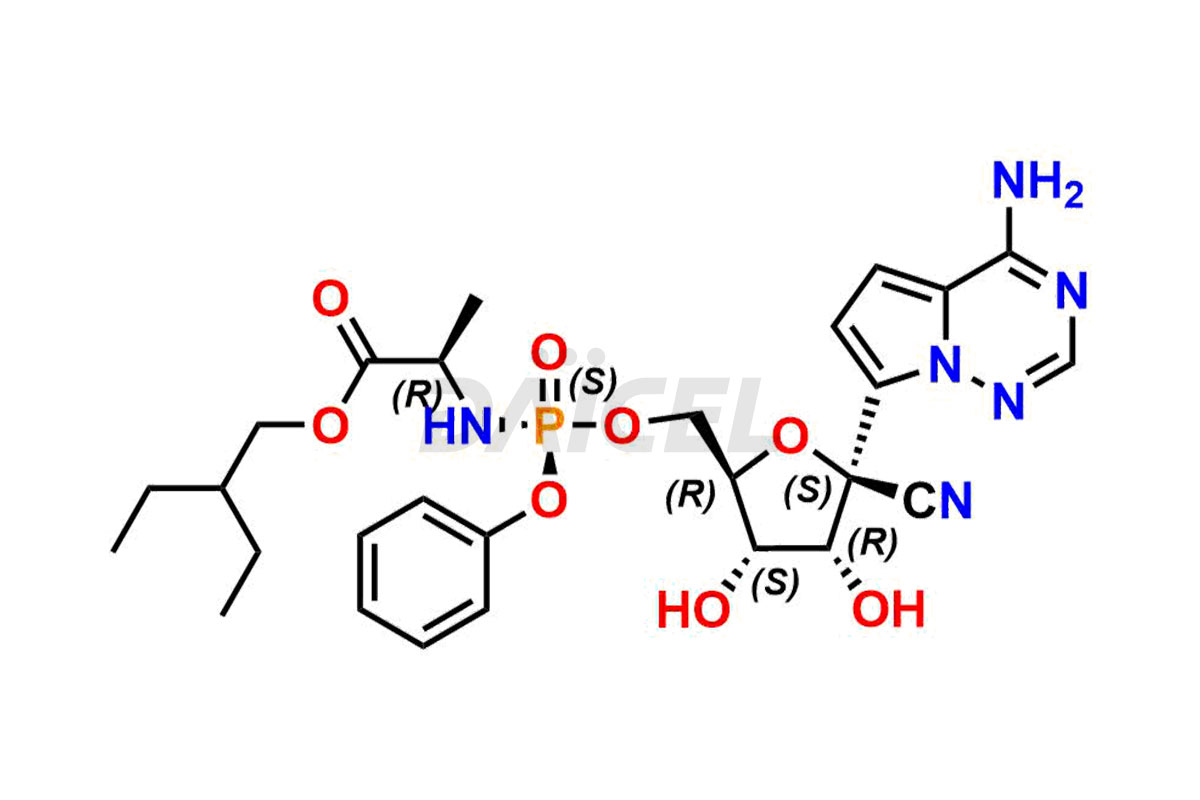
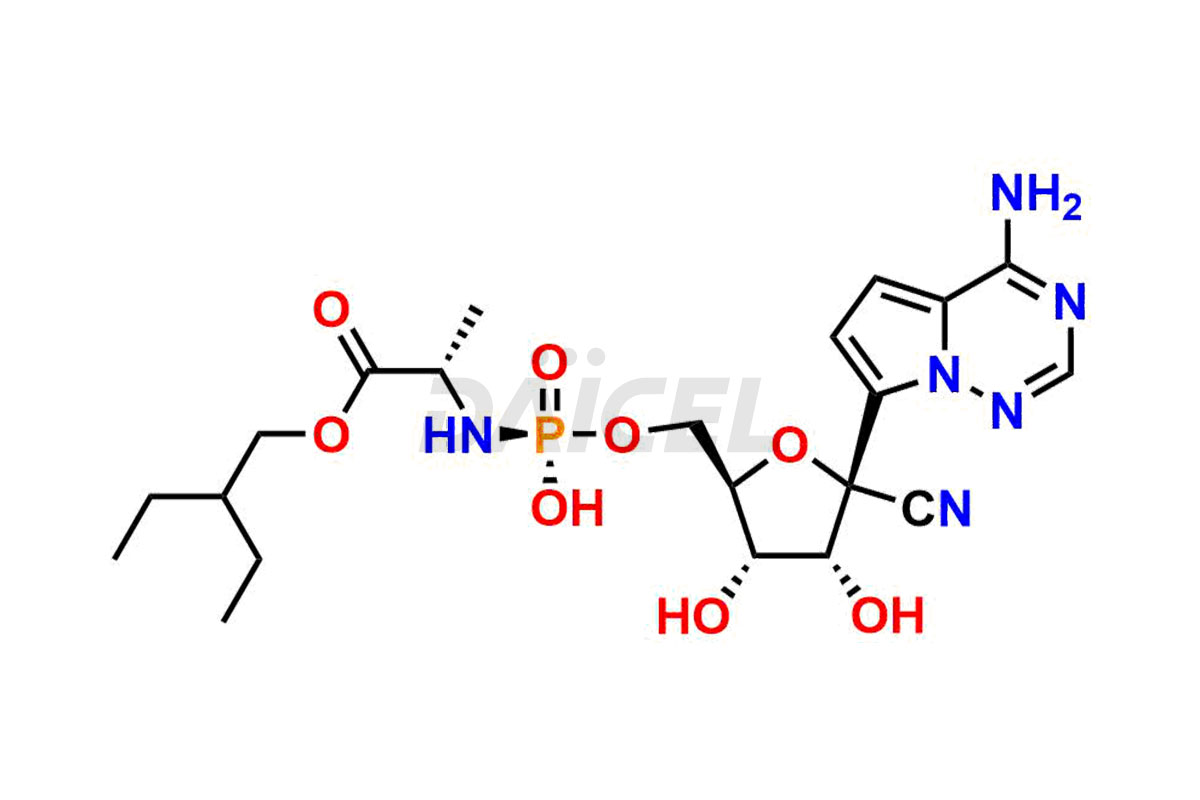
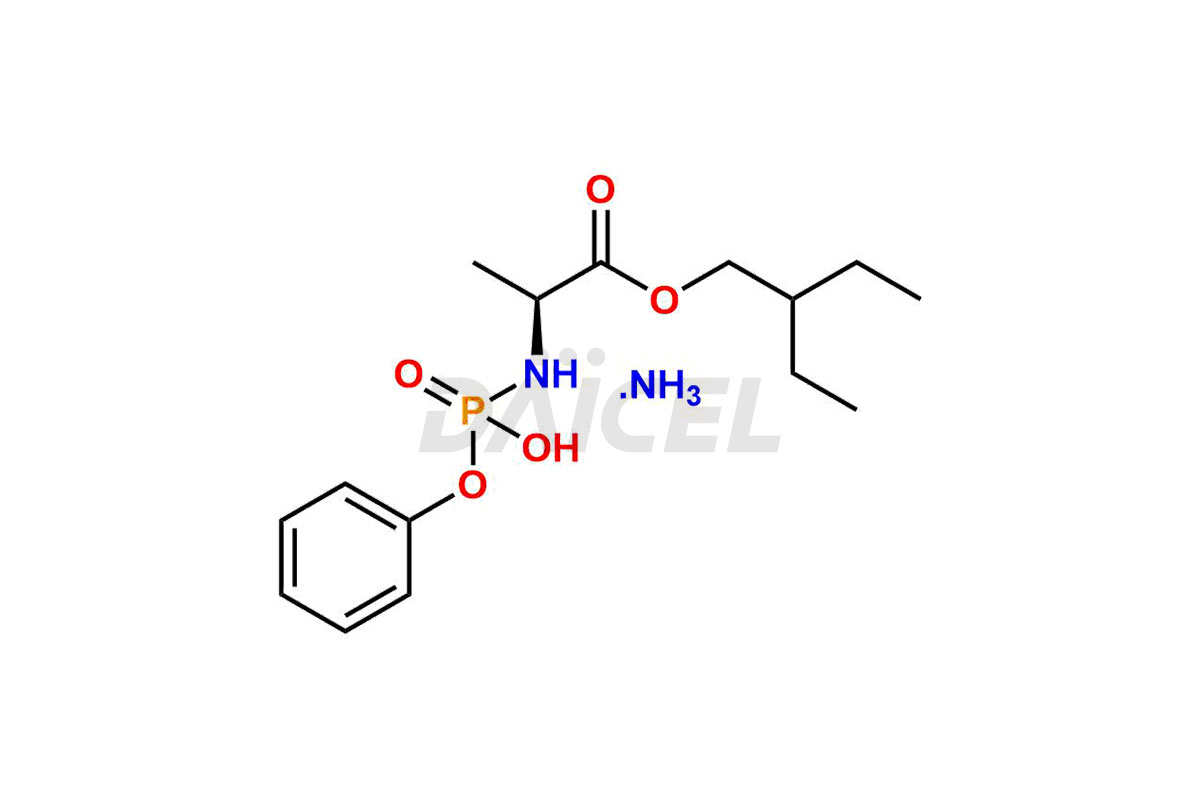
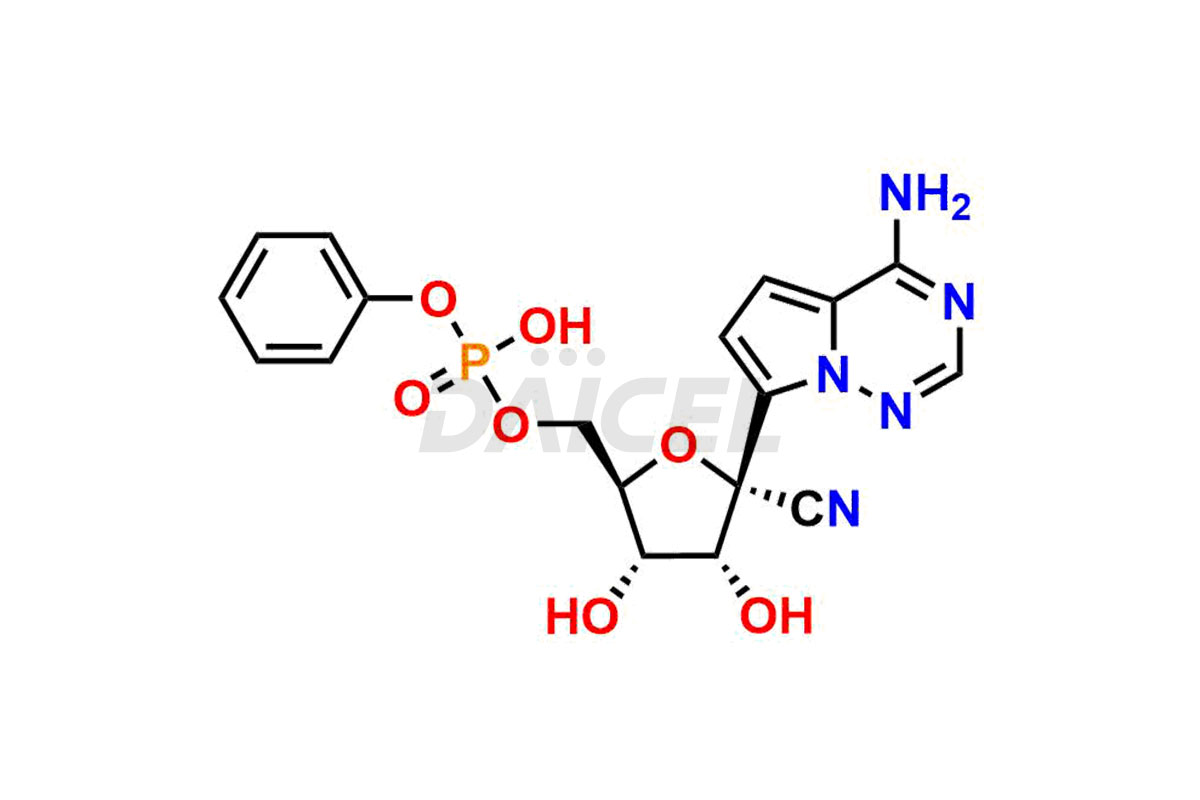
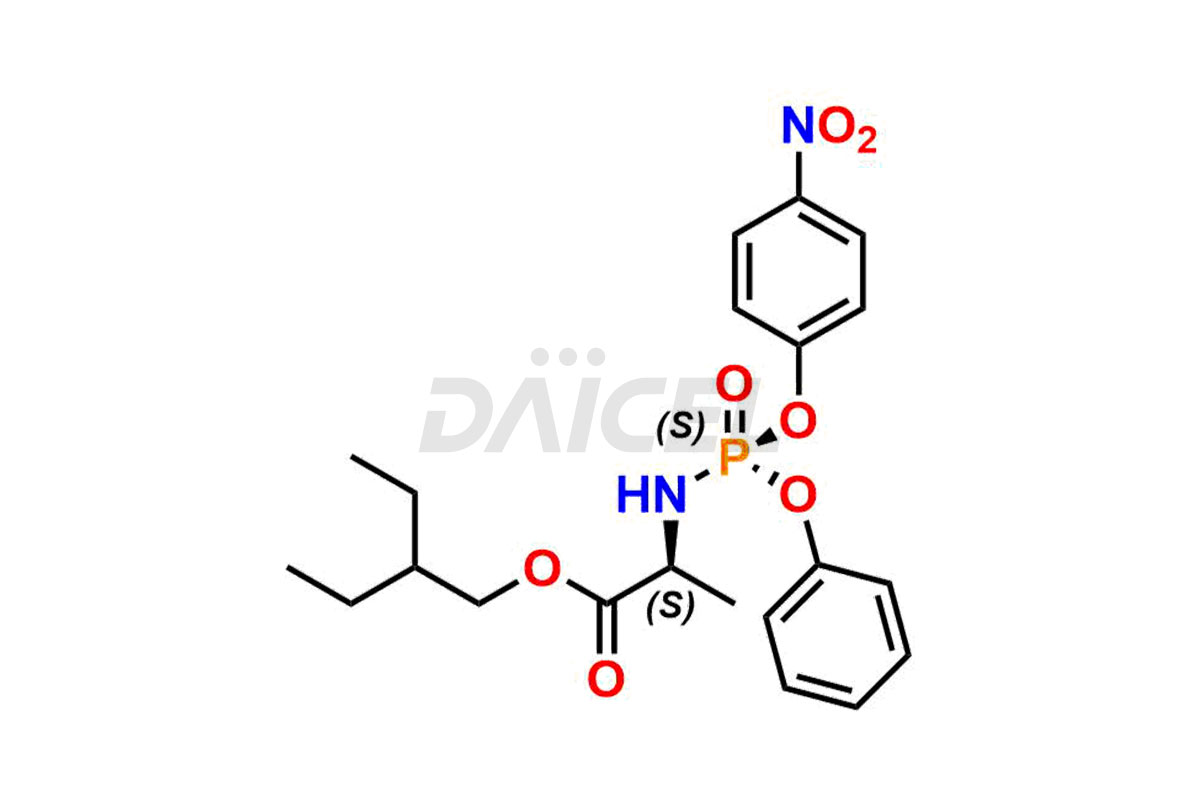
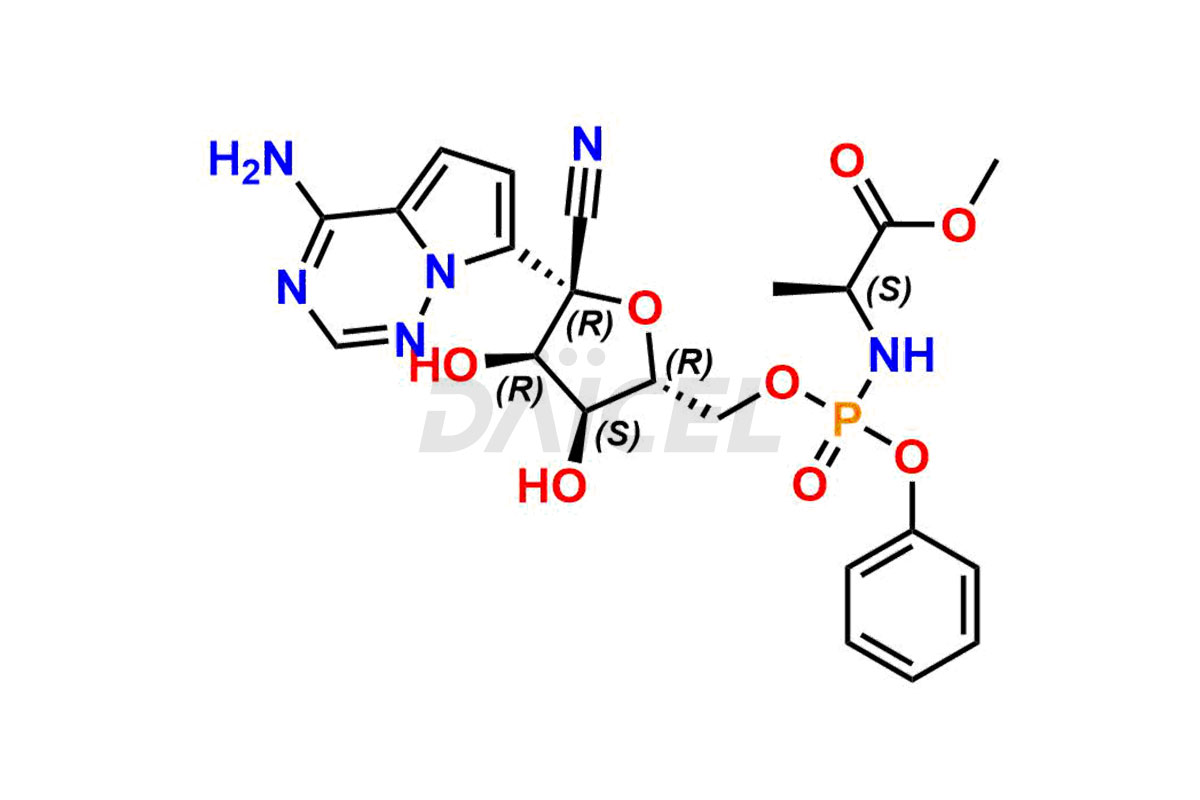
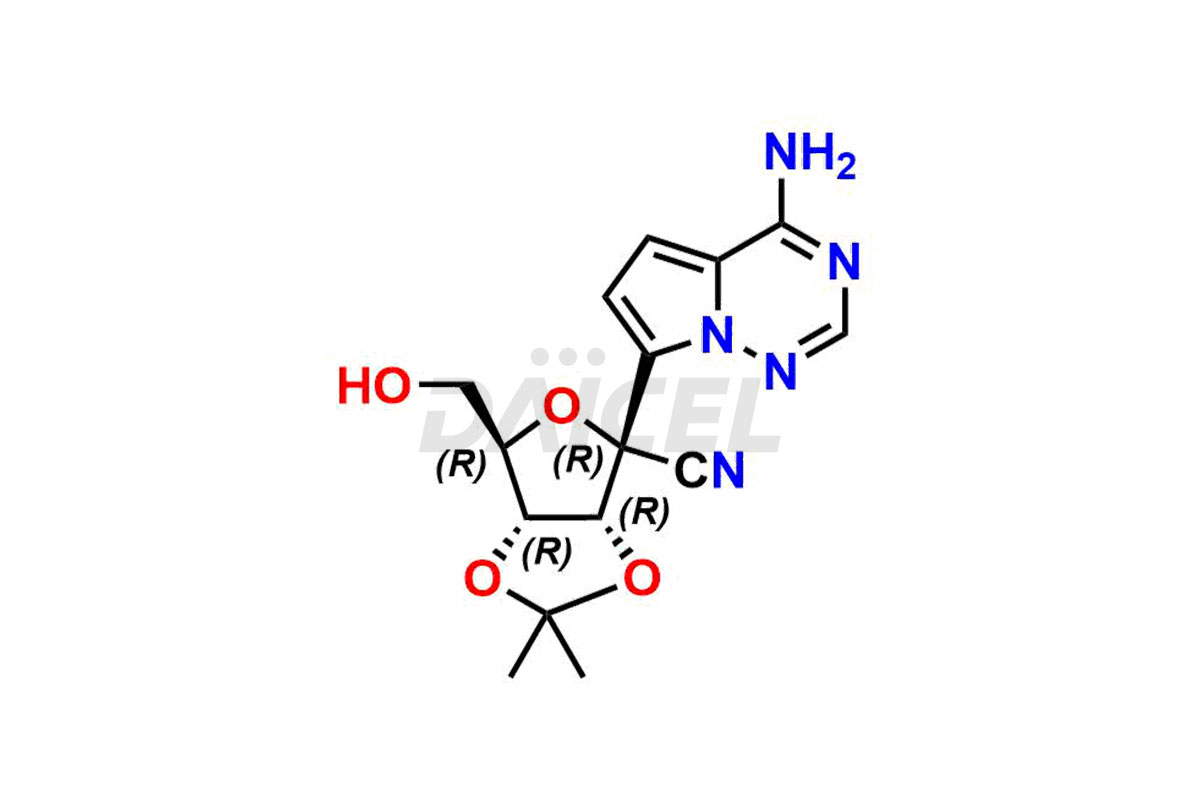
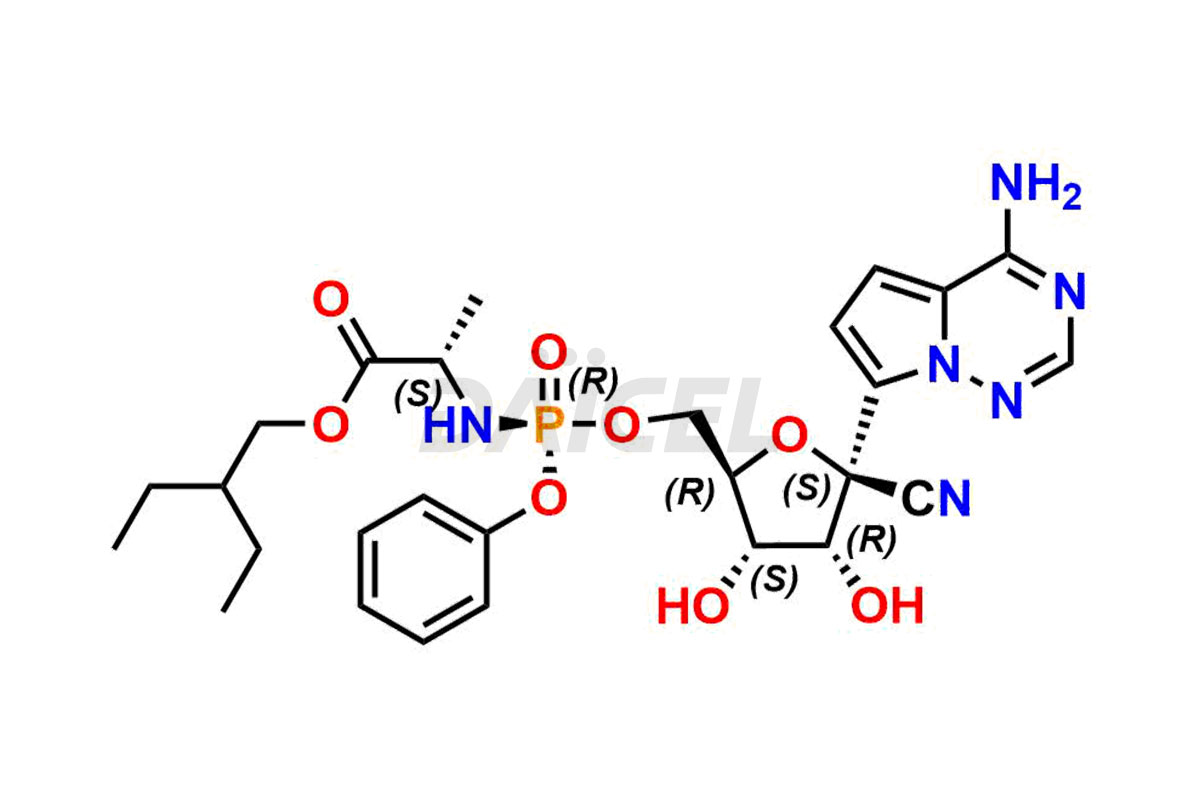
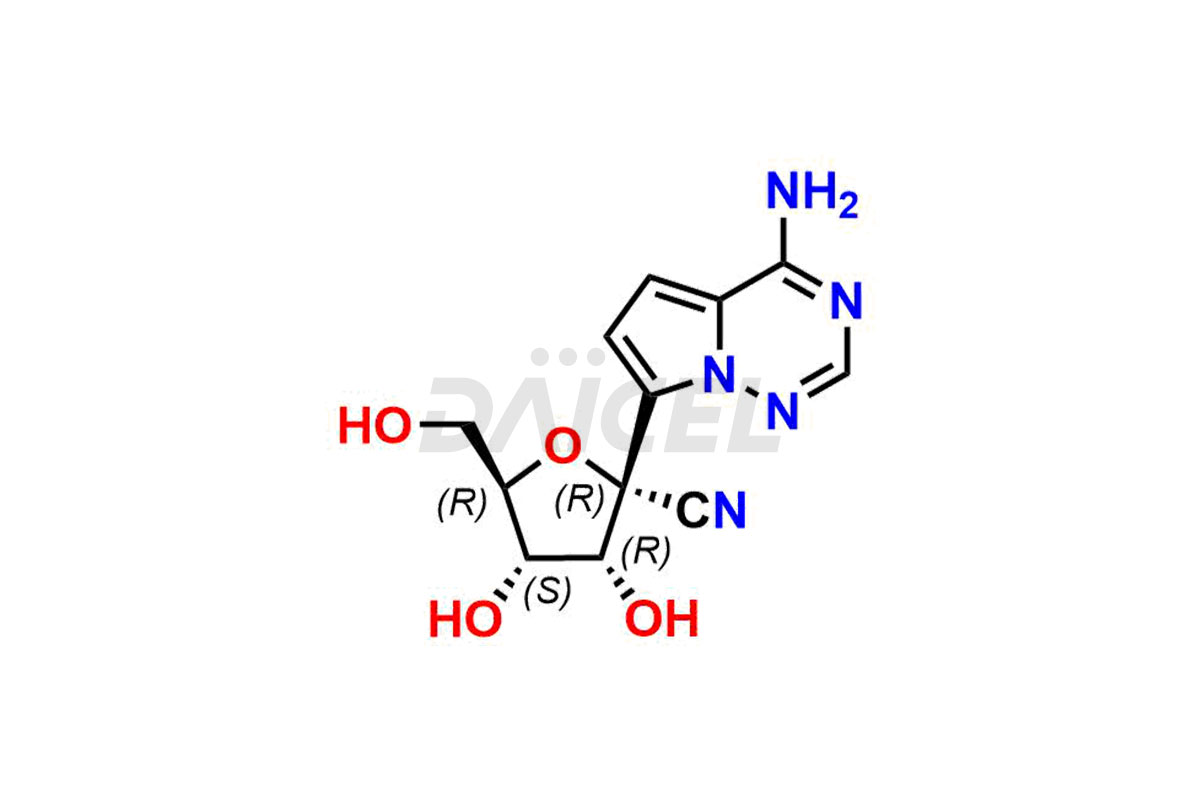
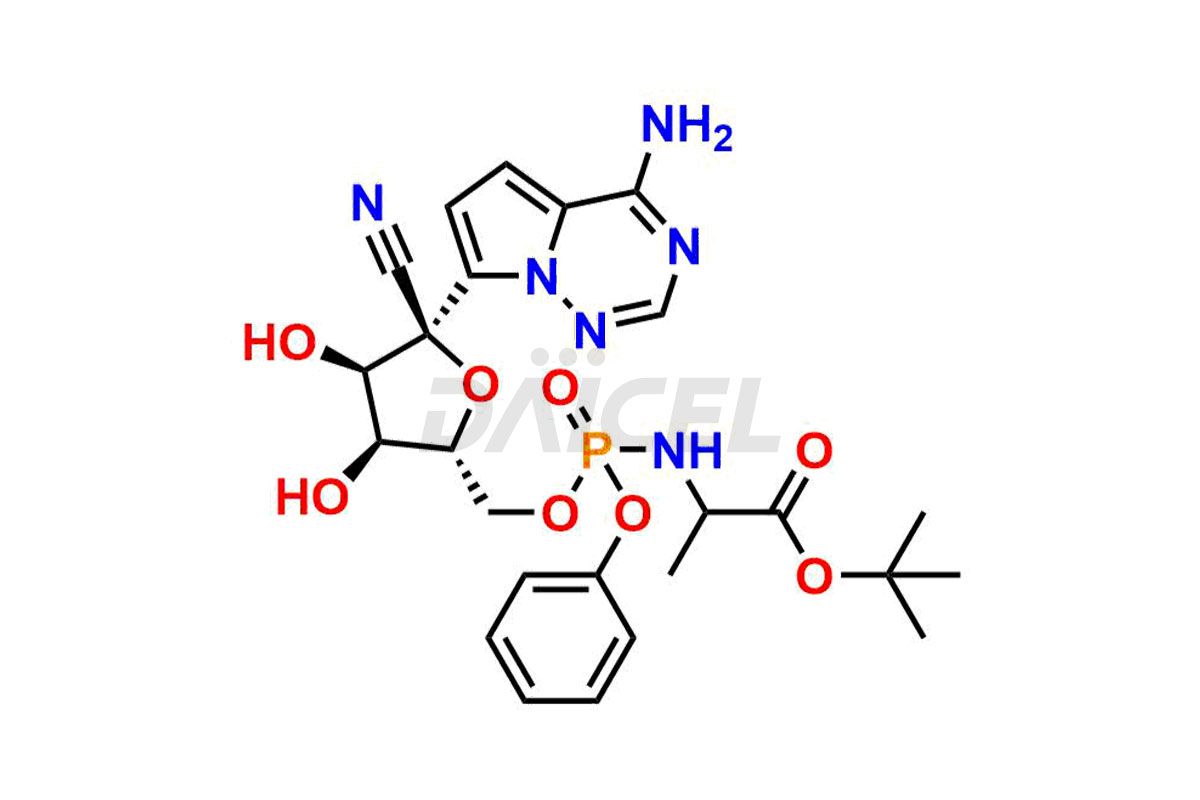
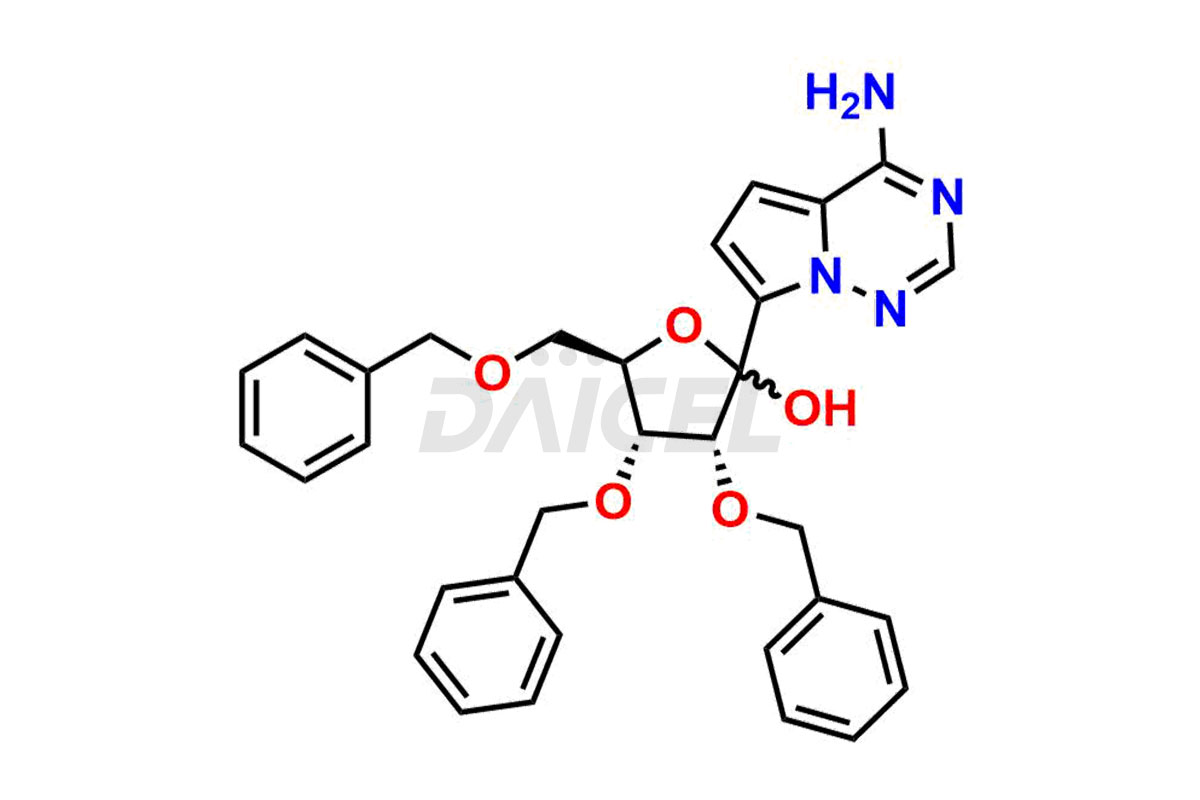
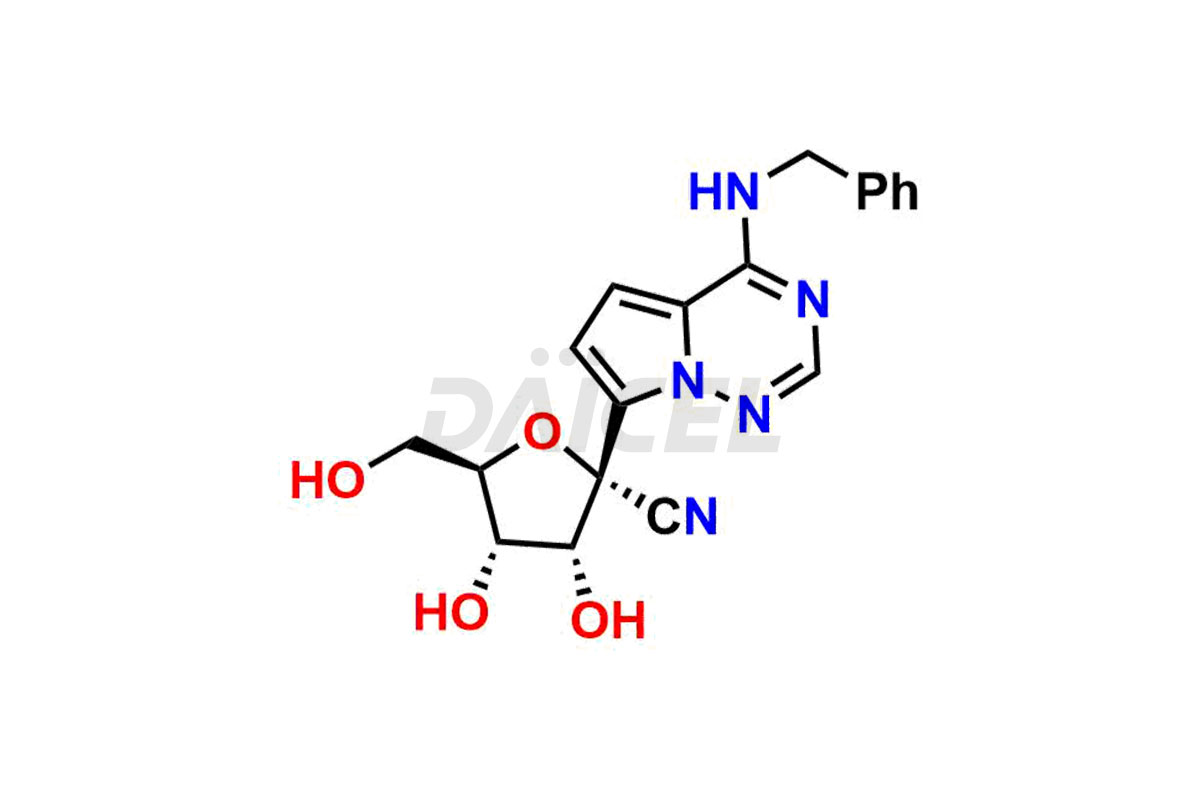
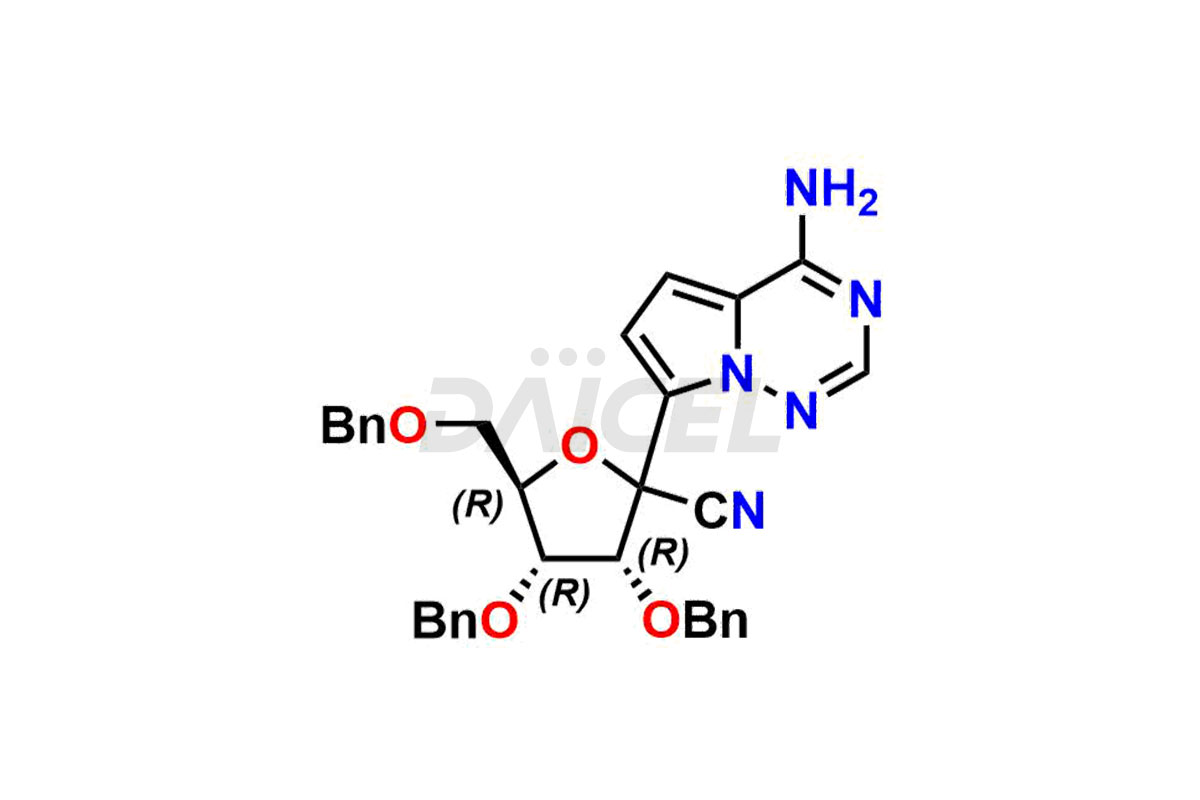
Remdesivir
General Information
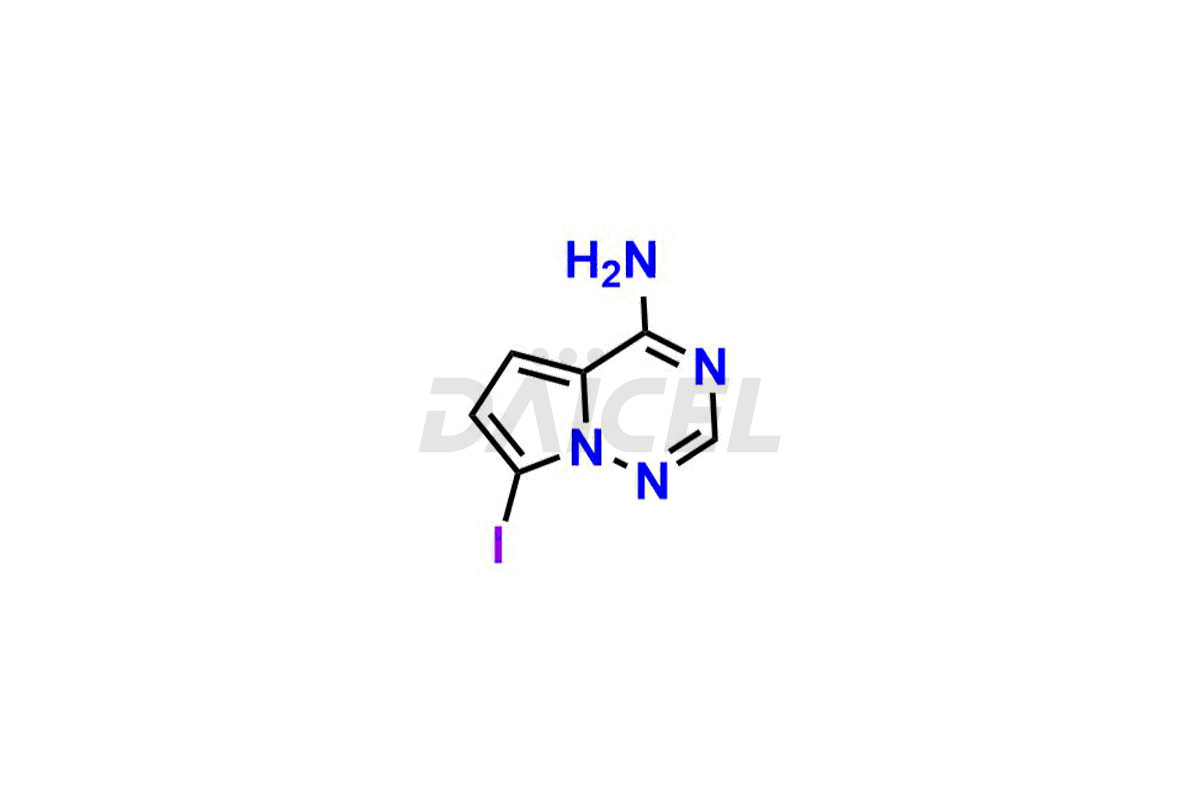
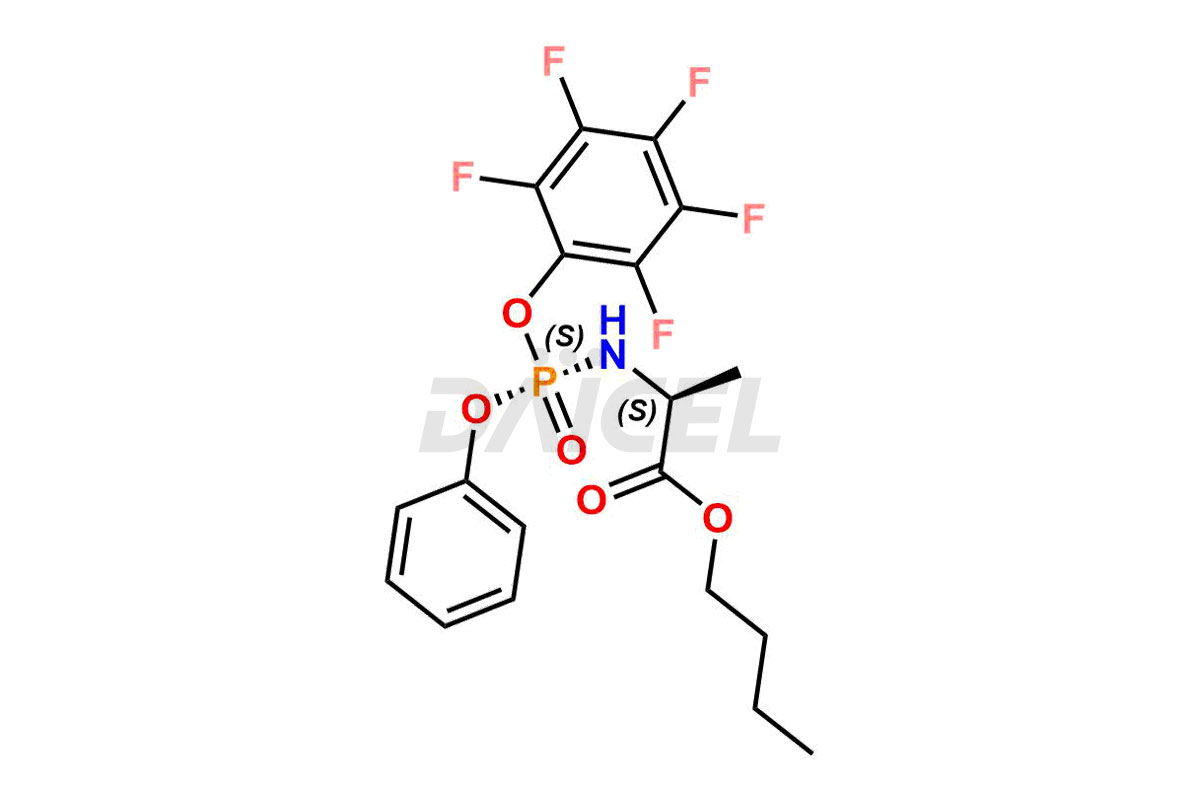
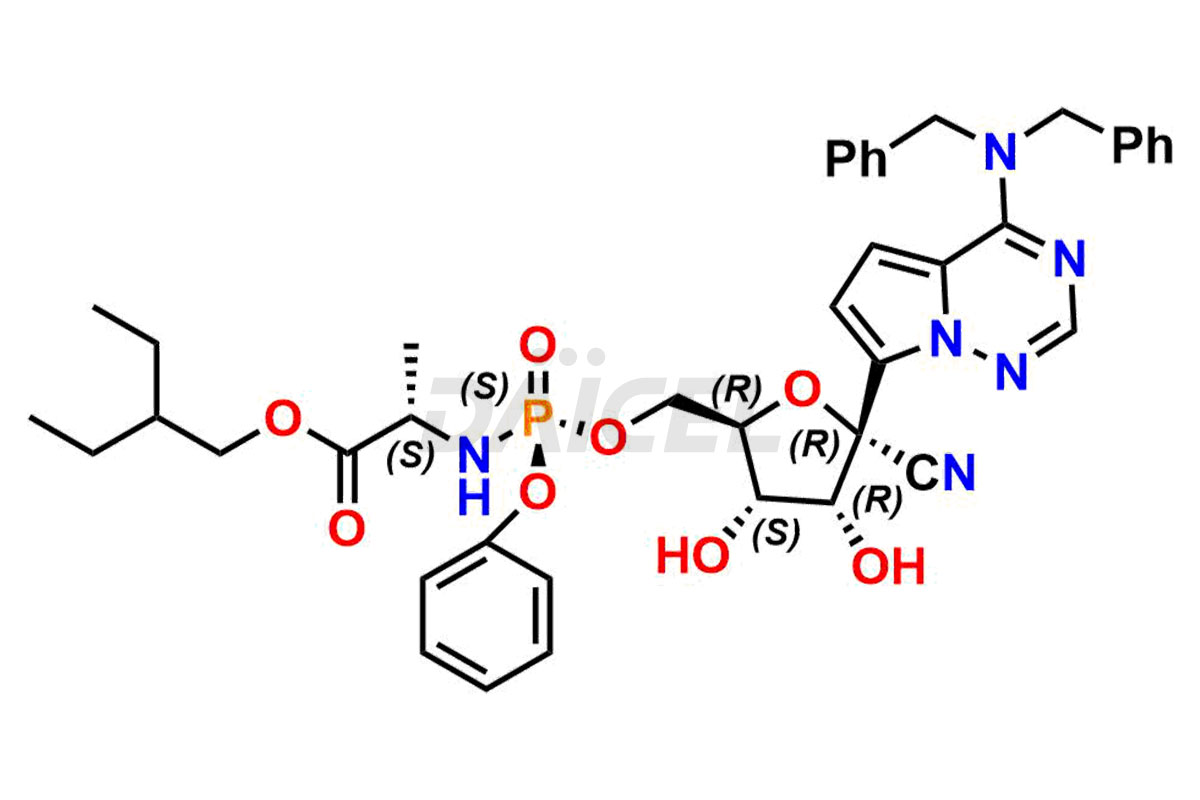
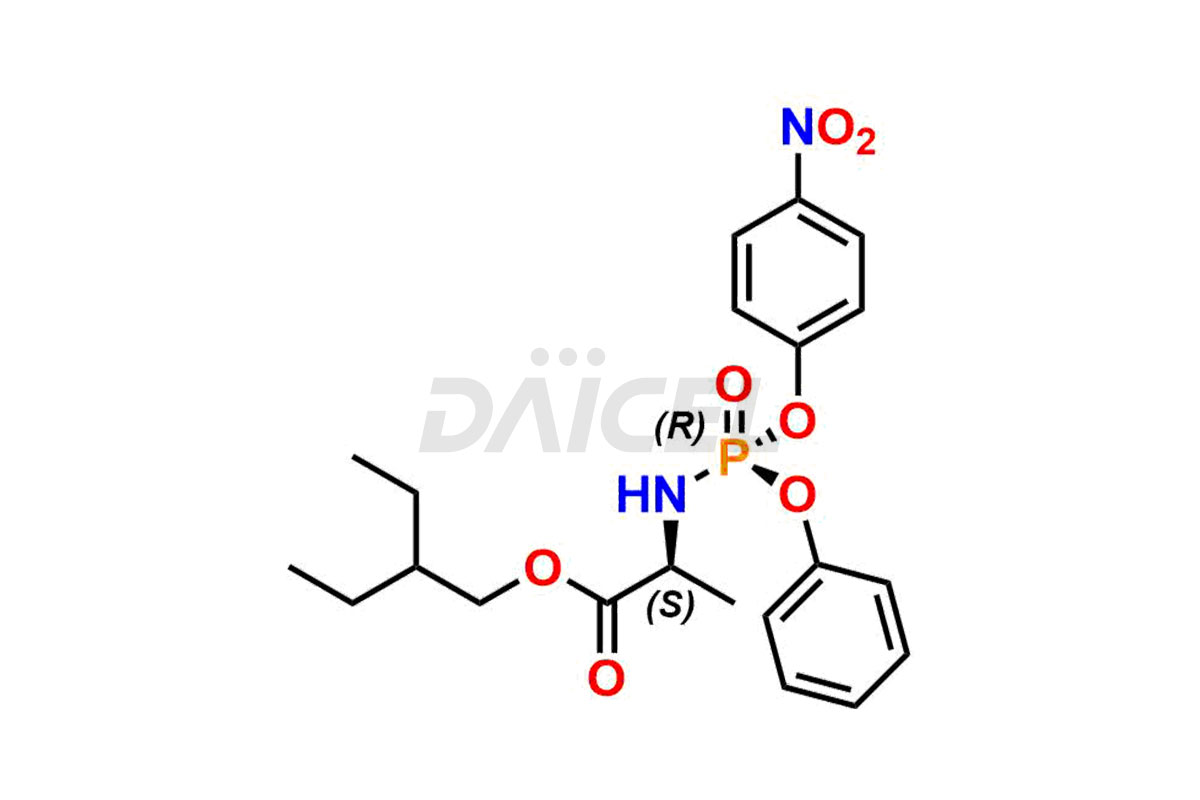
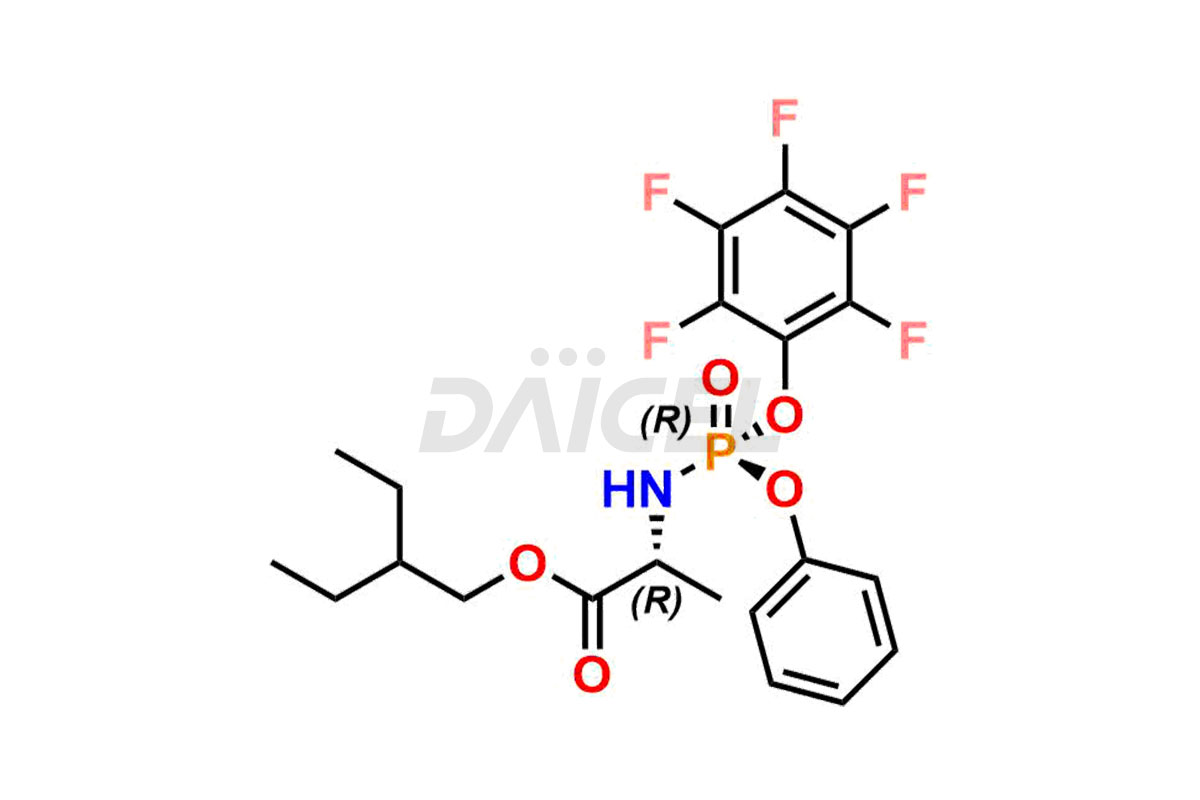
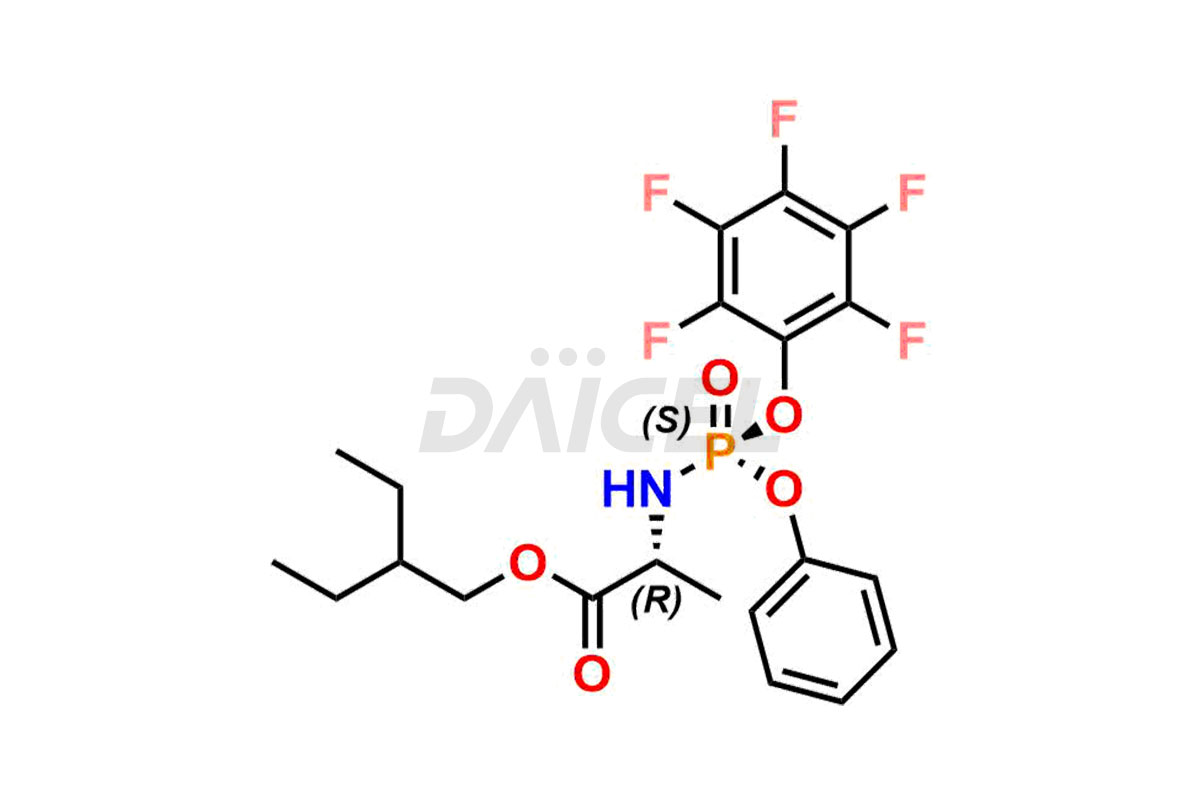
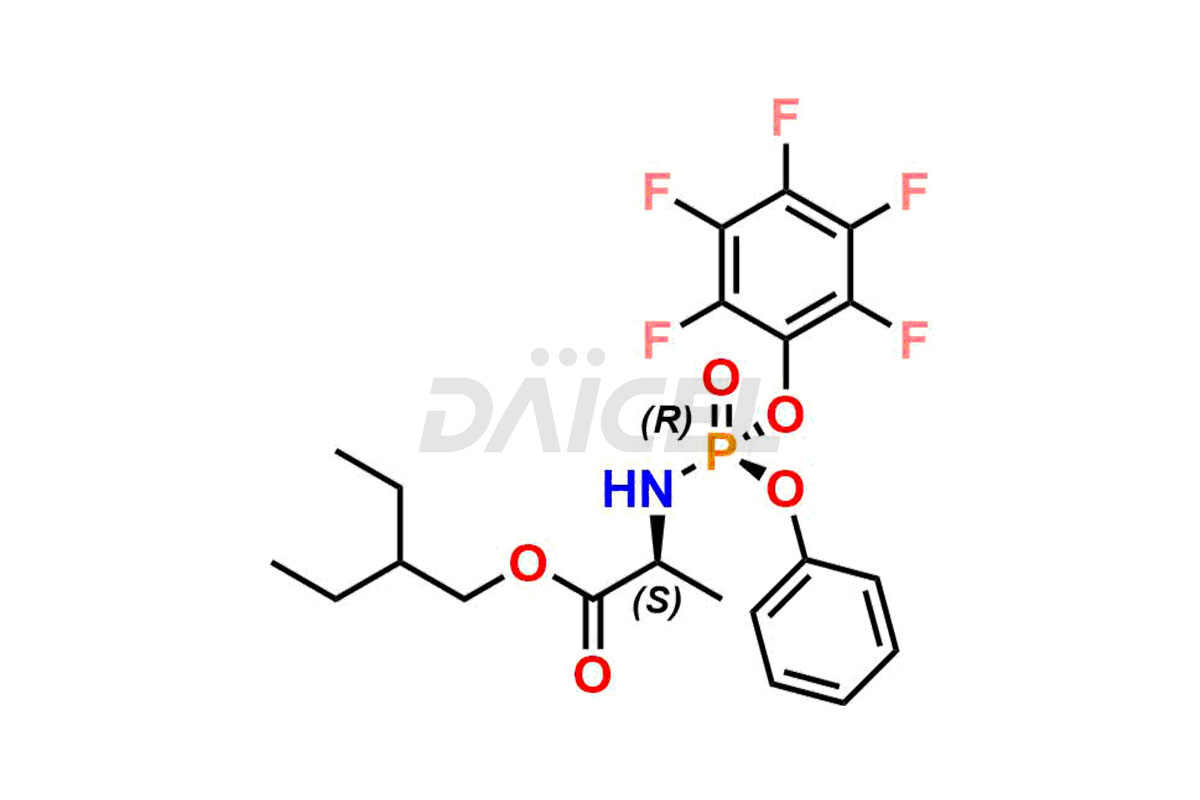
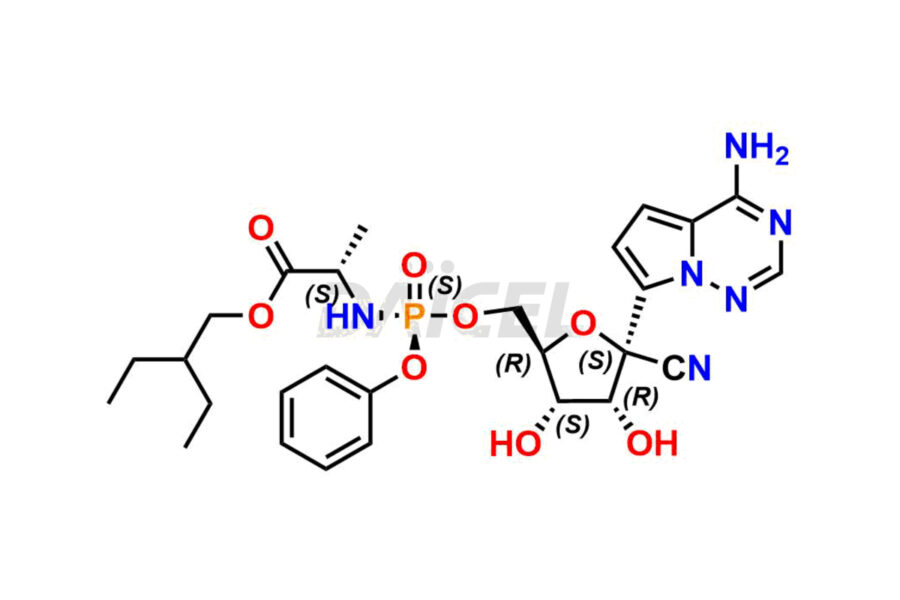
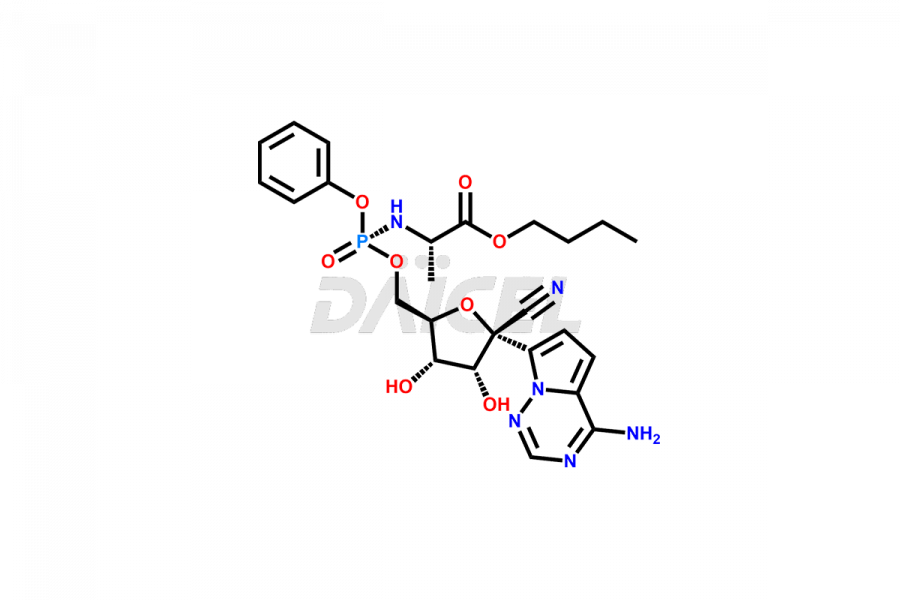
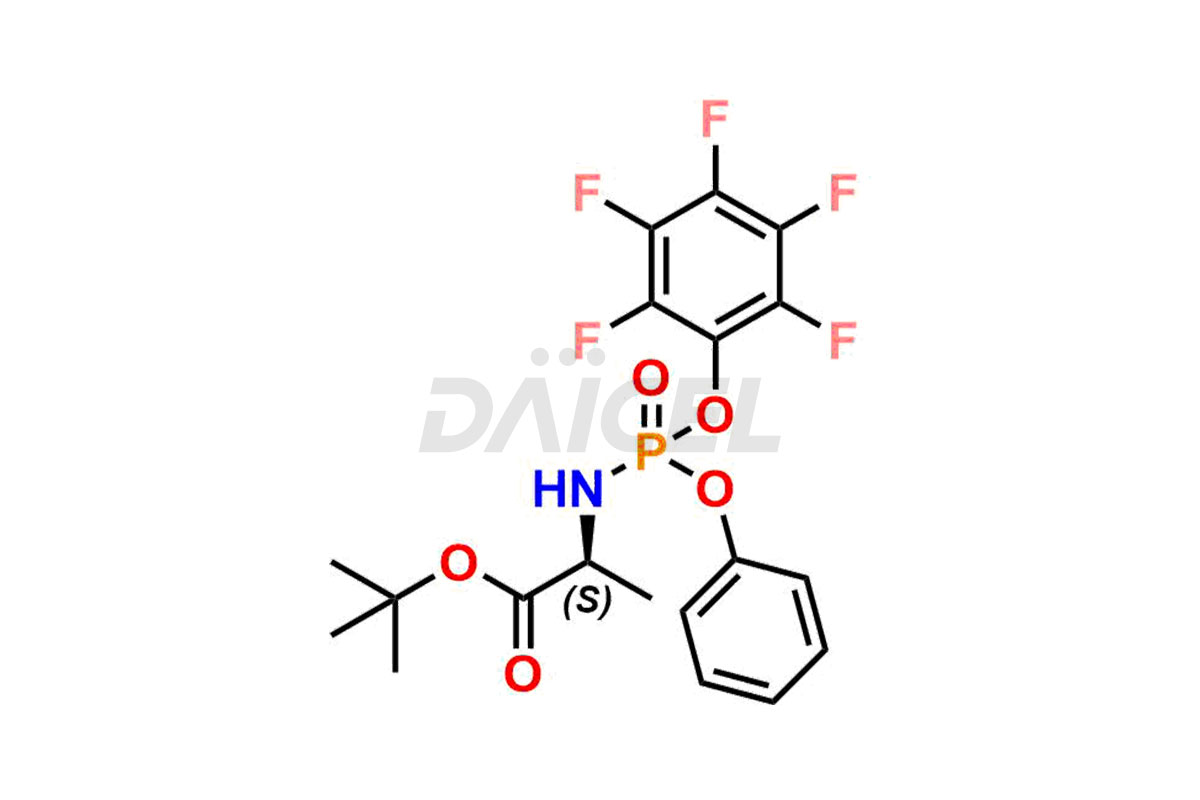
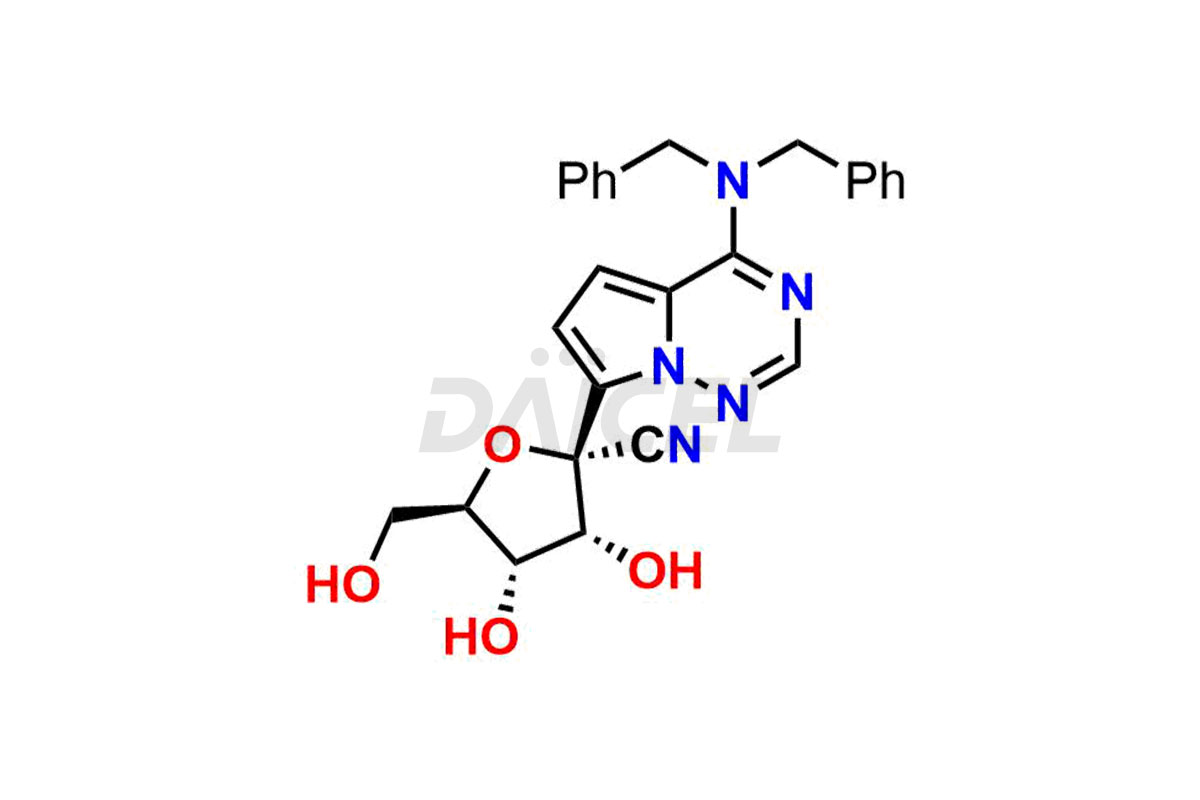
Remdesivir Impurities and Remdesivir
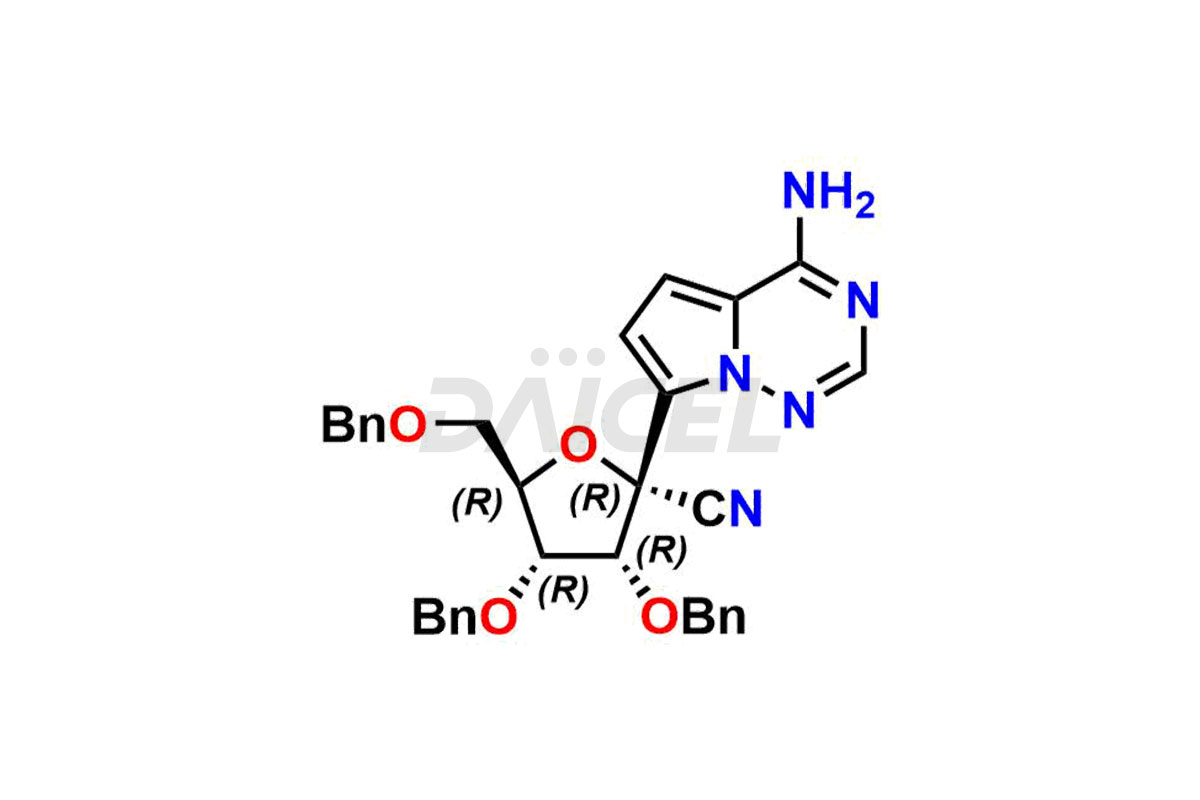
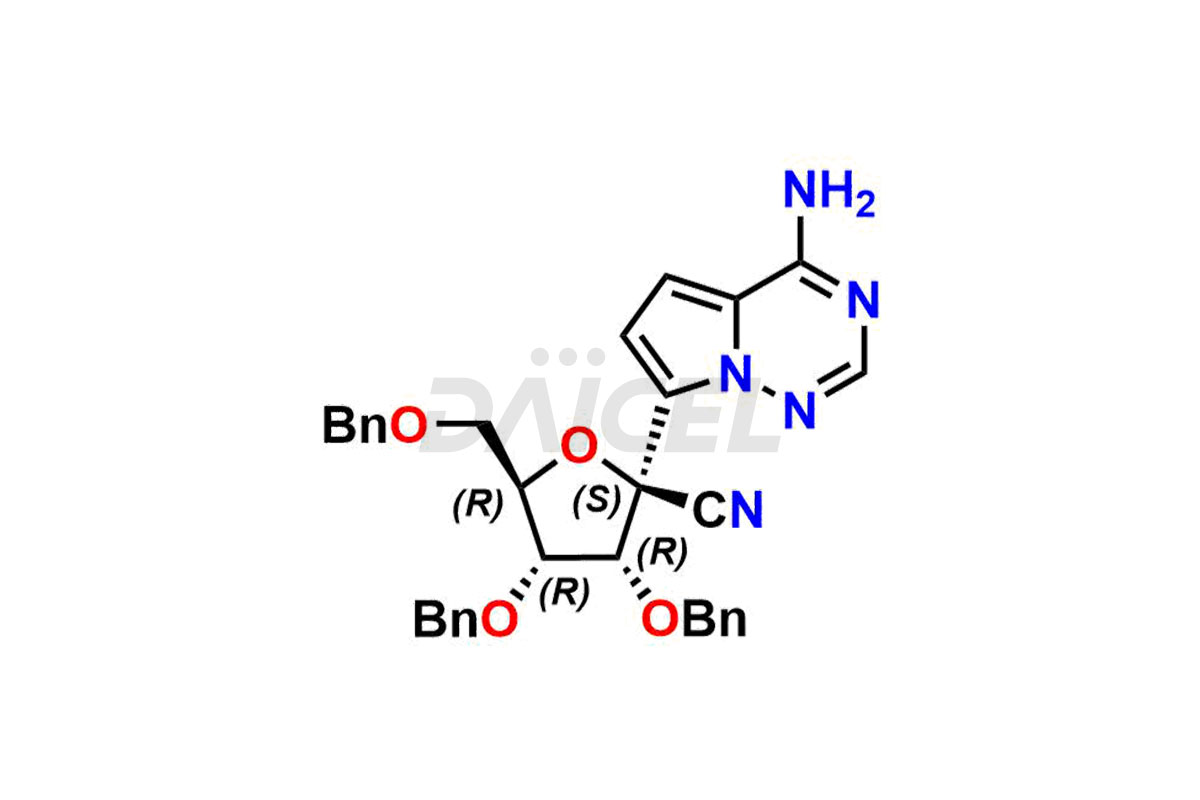
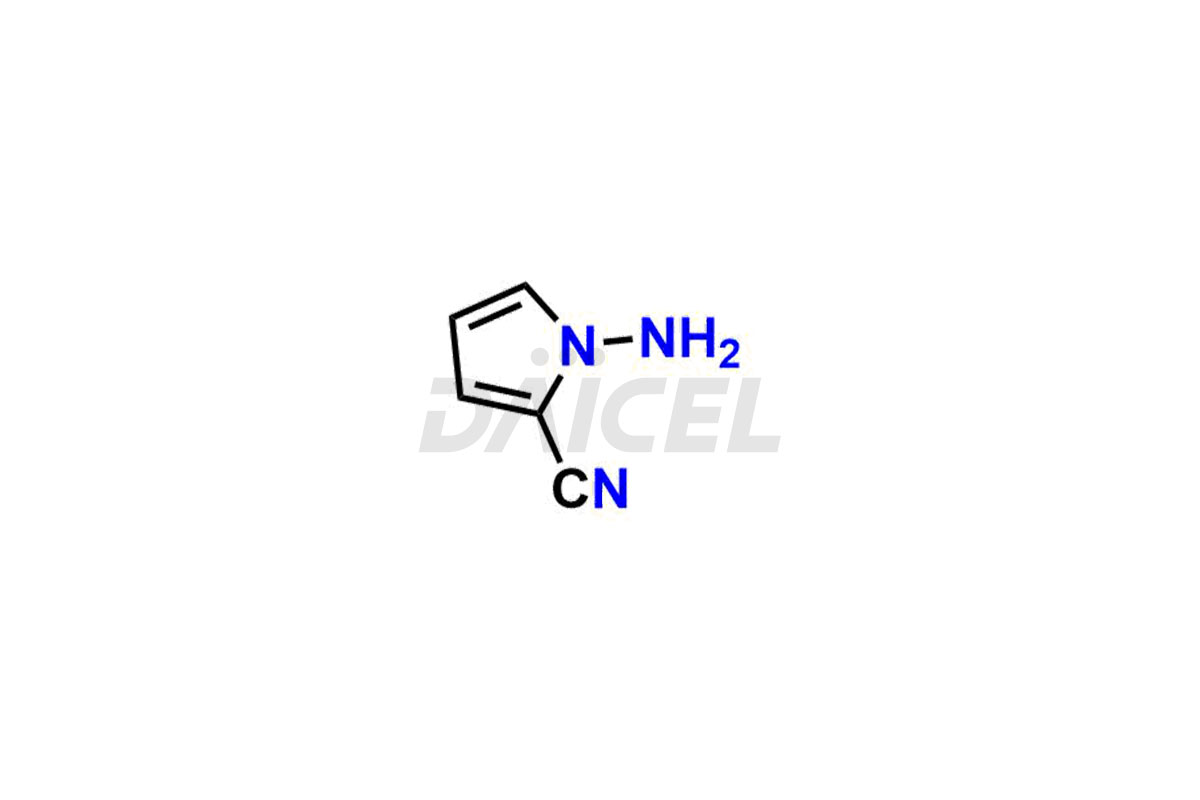
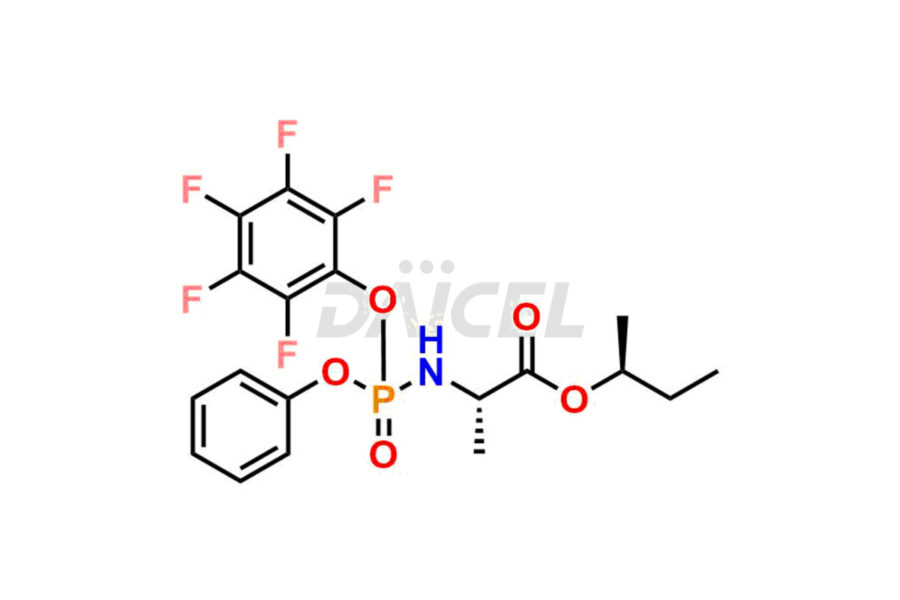
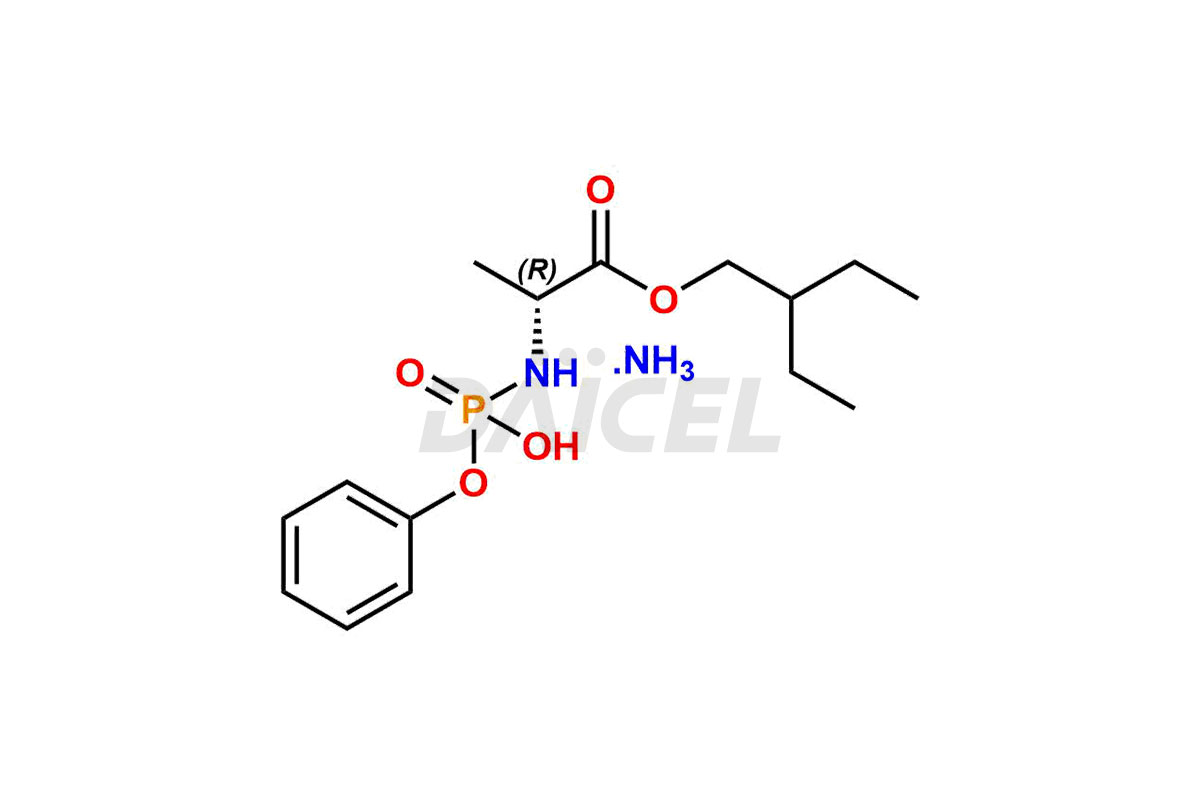
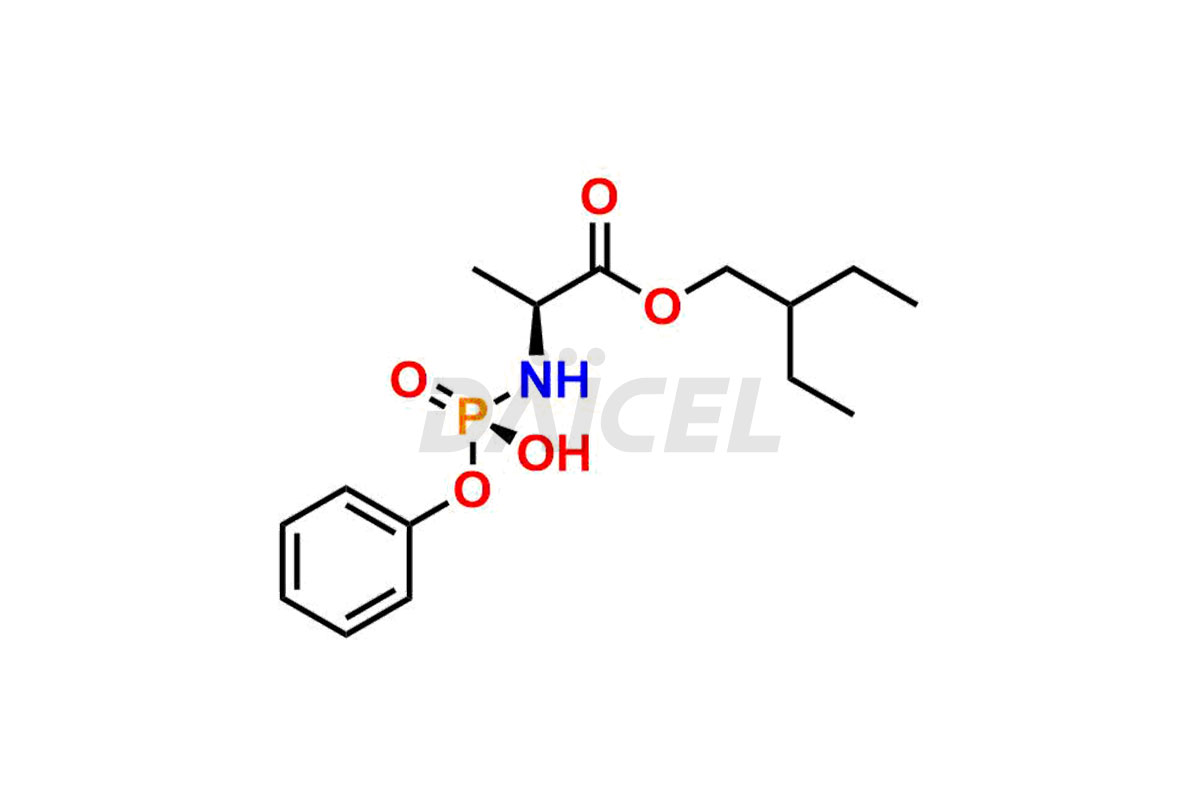
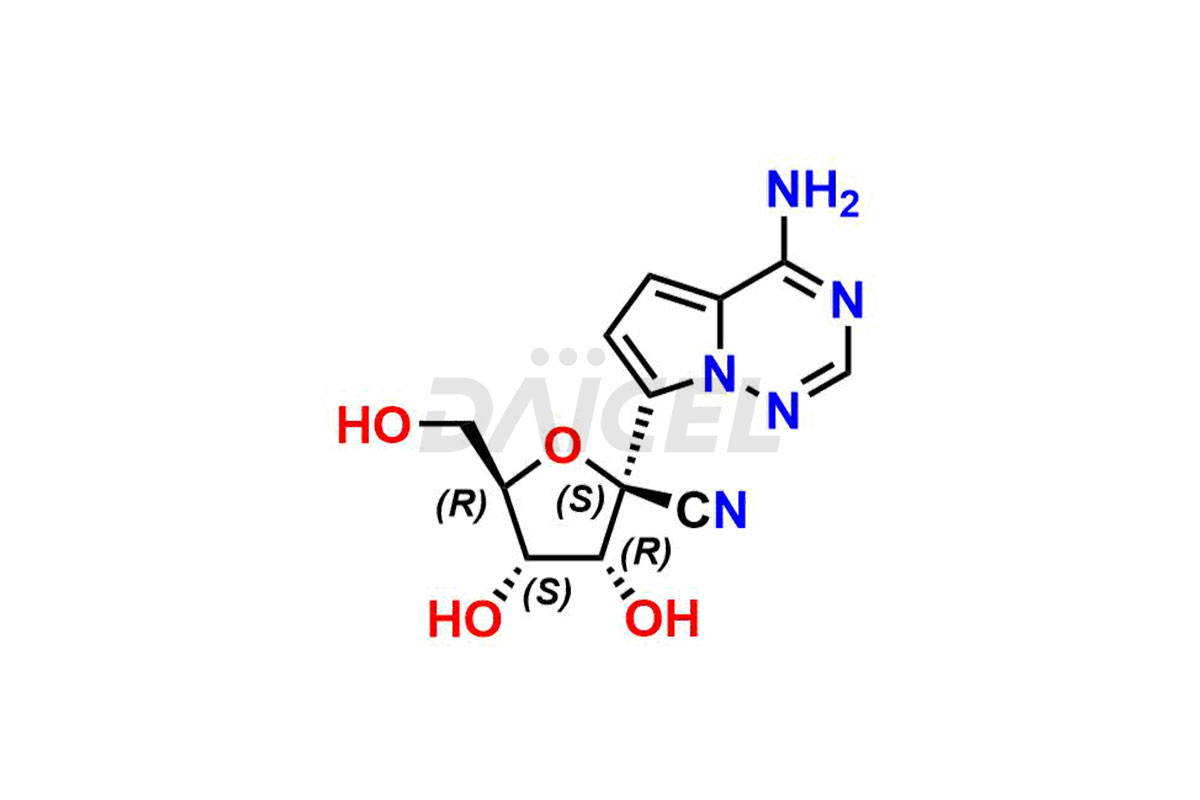
Daicel Pharma offers high-quality Remdesivir impurity standards, (R)-Tri O-benzyl nitrile Impurity, N-Butyl (SRR) Remdesivir, RDV pentafluoro RR isomer, Remdesivir Impurity- 1, Remdesivir Impurity 8, Remdesivir Impurity C, Remdesivir Impurity- 18, Remdesivir Impurity 14, Remdesivir R-P D-Alanine isomer, Remdesivir- S-Isomer at CN, and more. The effectiveness, stability, safety, and quality analysis of Remdesivir depends on these impurities. Daicel Pharma offers custom synthesis for Remdesivir impurities and delivers them internationally.
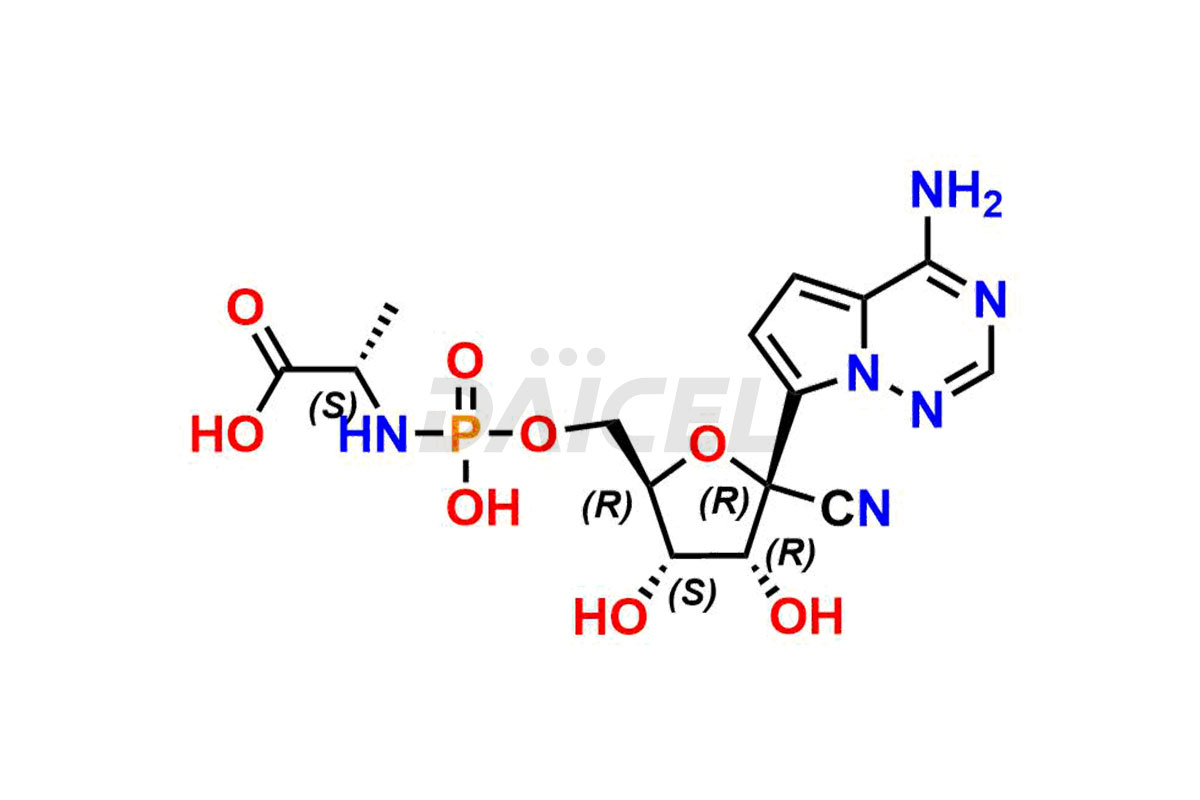
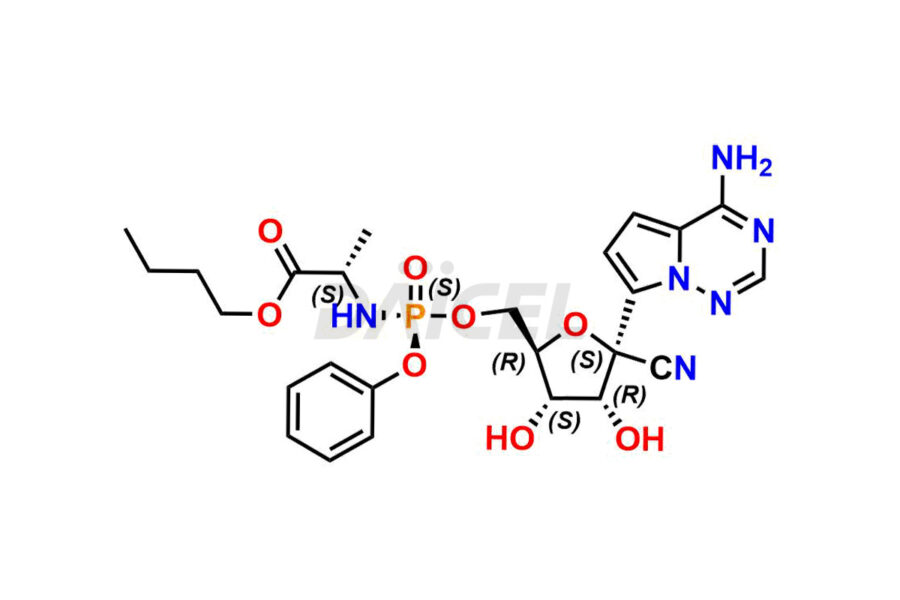
Remdesivir [CAS: 1809249-37-3] is an antiviral medication for treating RNA virus infections. It is a SARS-CoV-2 nucleotide analog RNA polymerase inhibitor.
Remdesivir: Use and Commercial Availability
Remdesivir treats mild-to-moderate COVID-19 patients at high risk of severe COVID-19, including hospitalization or death.
This medication is available under the brand name Veklury.
Remdesivir Structure and Mechanism of Action 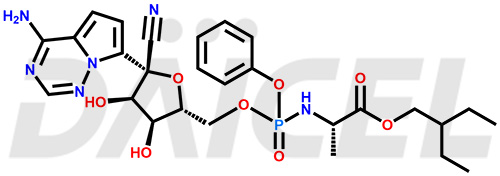
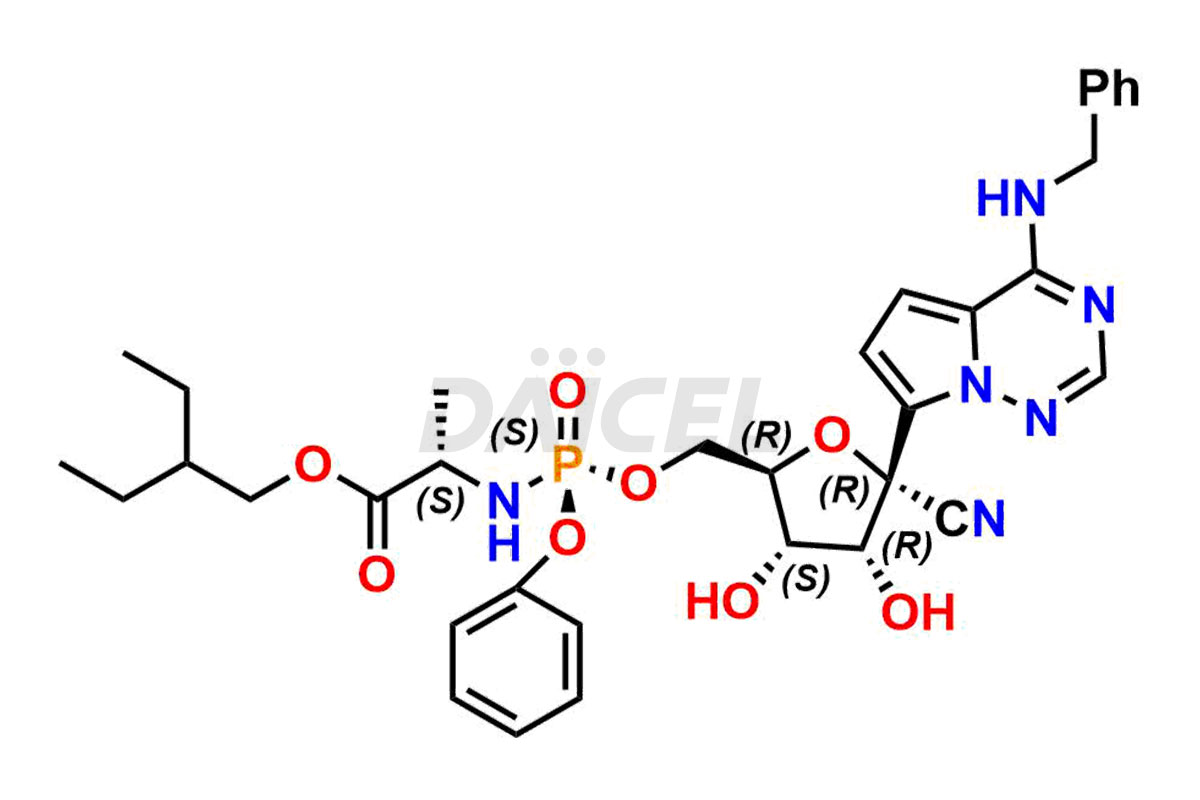
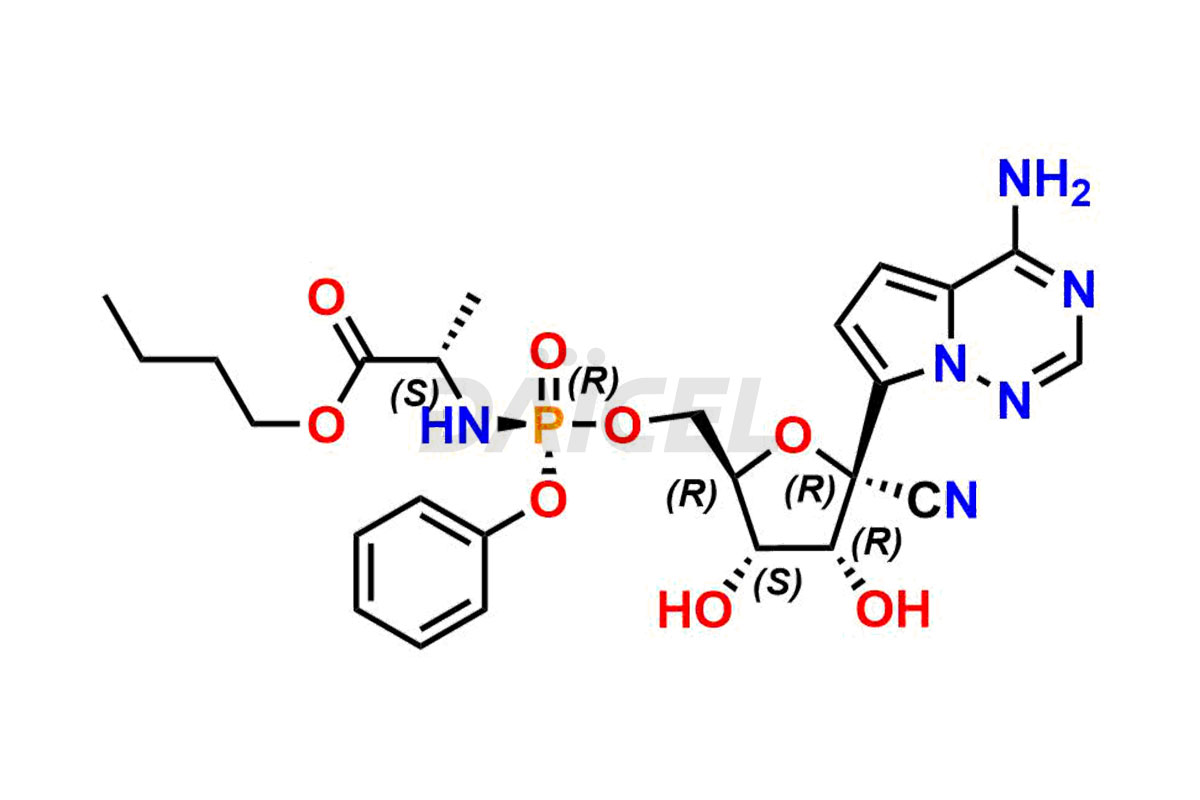
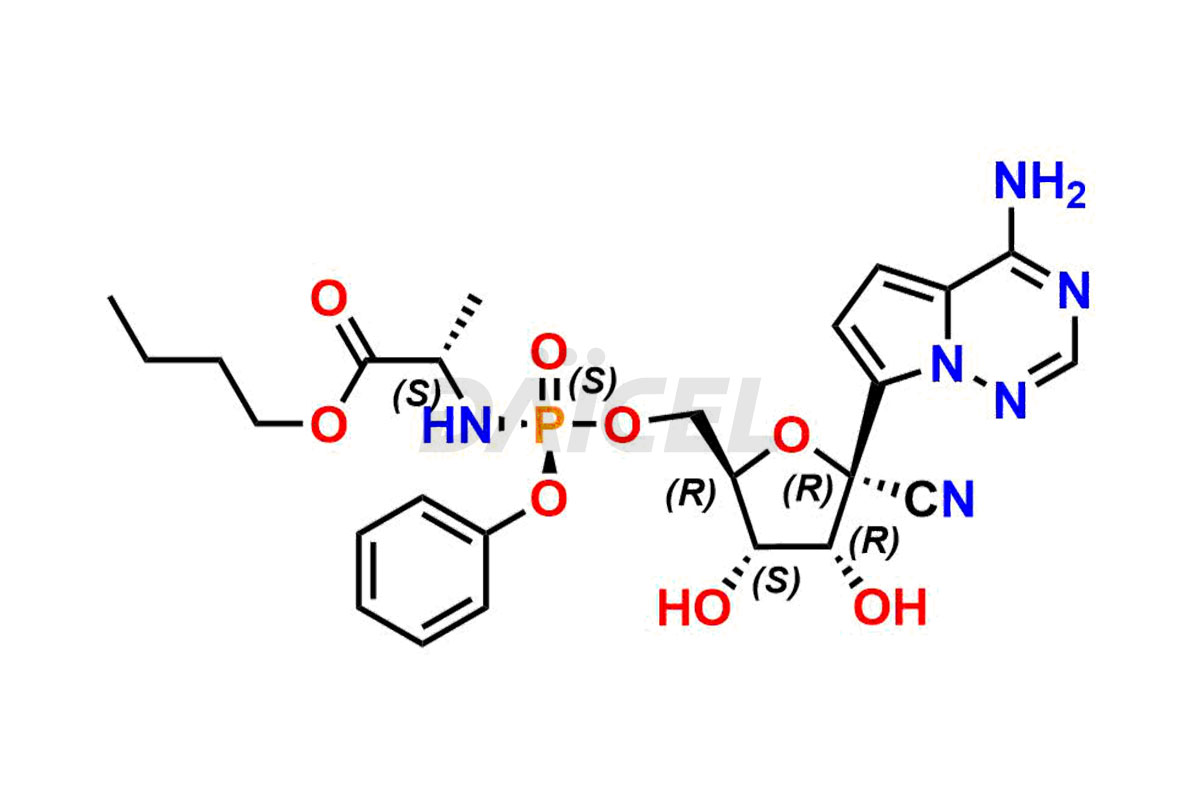
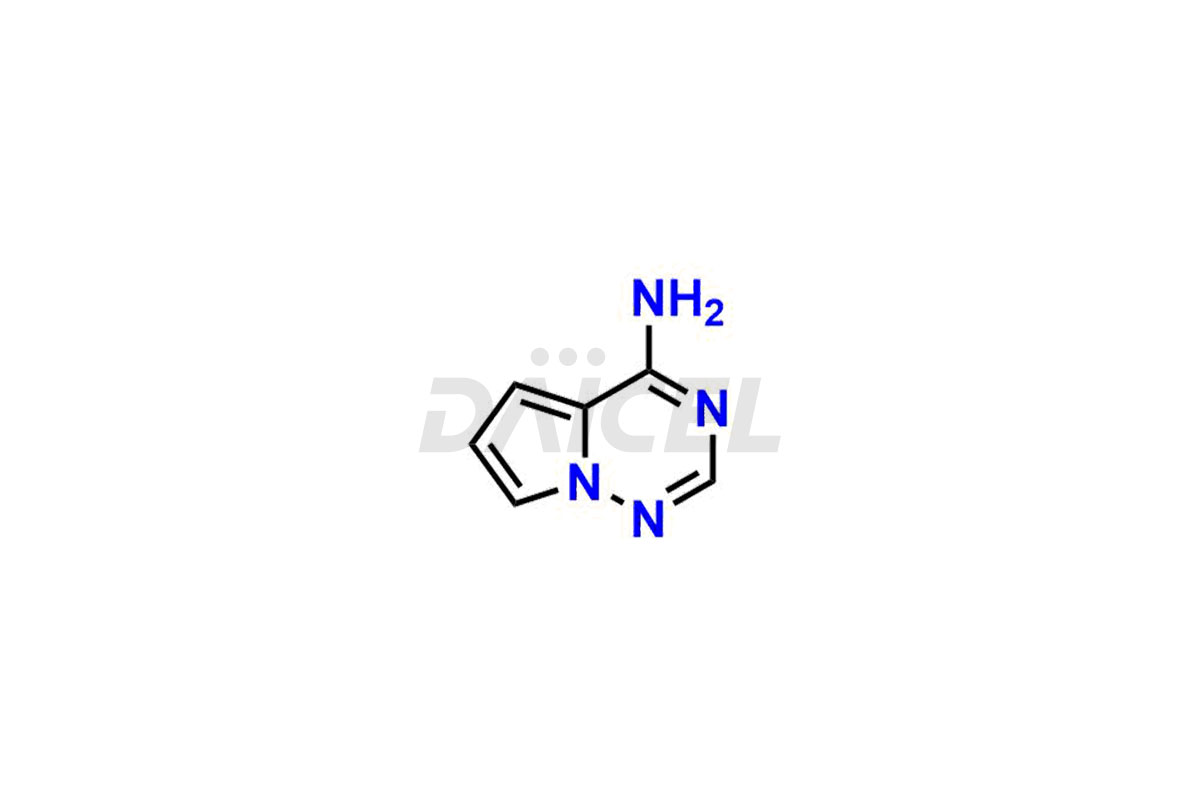
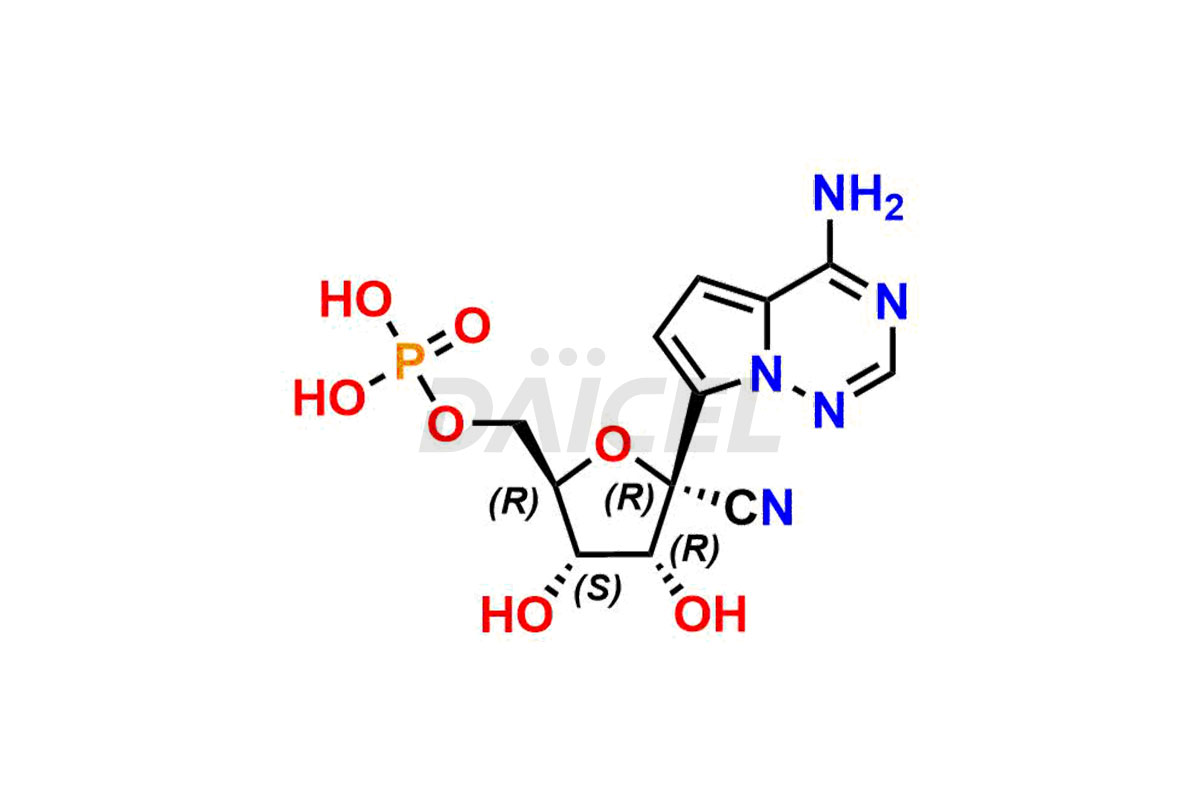
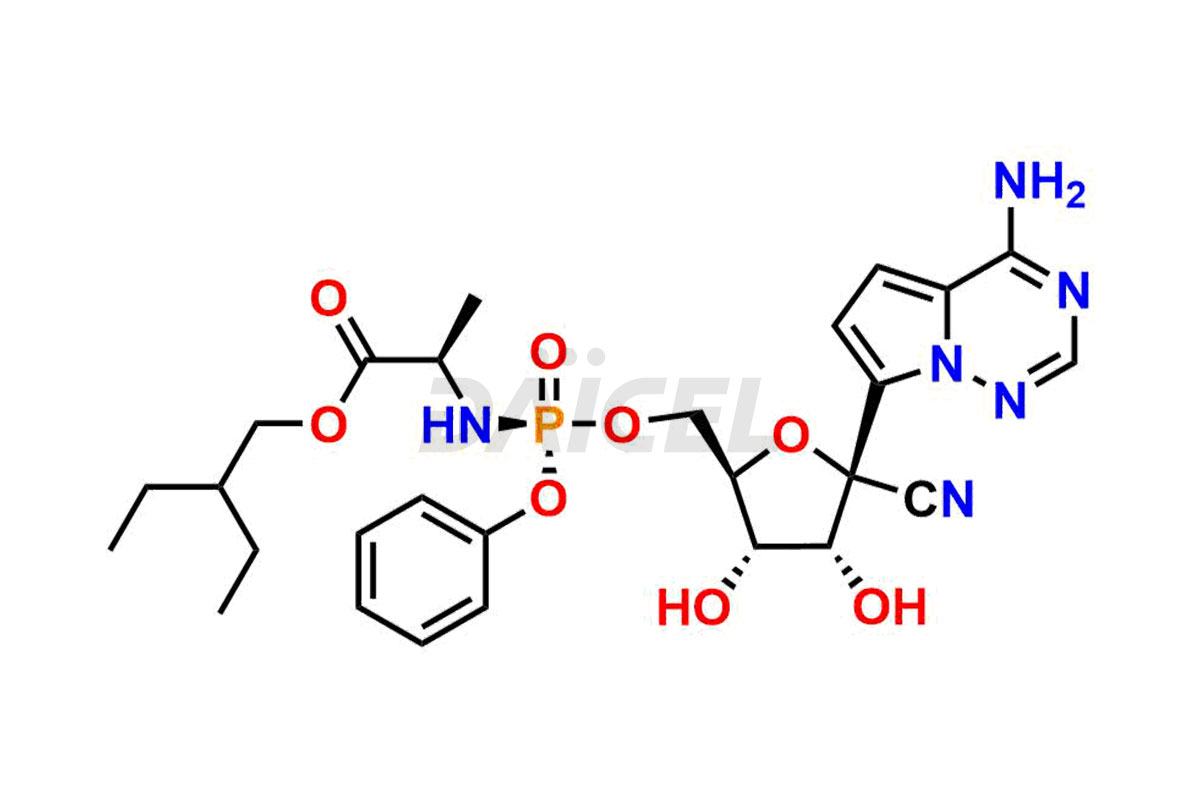
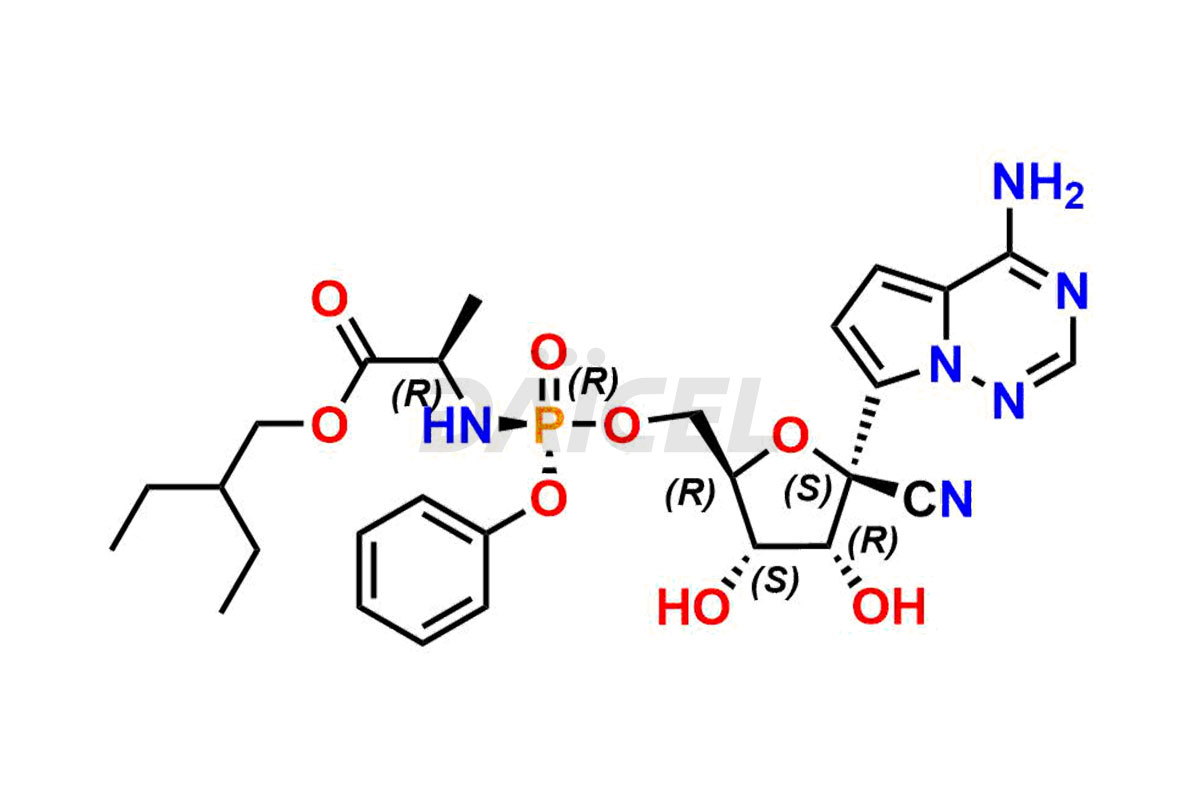
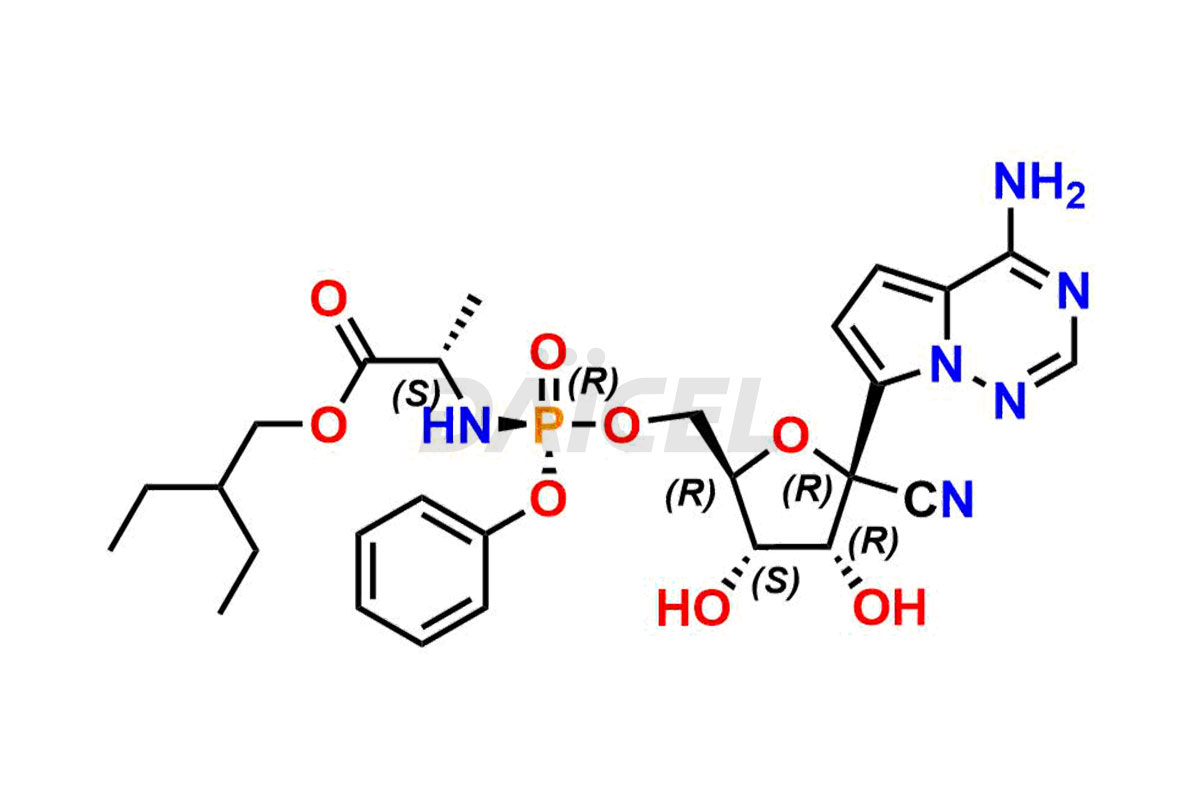
The chemical name of Remdesivir is (S)-2-Ethylbutyl 2-(((S)-(((2R,3S,4R,5R)-5-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-5-cyano-3,4-dihydroxytetrahydrofuran-2-yl)methoxy)(phenoxy) phosphoryl)amino)propanoate. Its chemical formula is C27H35N6O8P, and its molecular weight is approximately 602.6 g/mol.
Remdesivir inhibits the SARS-CoV-2 RNA-dependent RNA polymerase (RdRp) and blocks viral RNA synthesis.
Remdesivir Impurities and Synthesis
Remdesivir impurities are unintended chemical compounds present during the synthesis1 or storage of Remdesivir, an antiviral medication for treating viral infections such as COVID-19. These impurities can arise from various sources, including starting materials, reagents, intermediates, or degradation products.
Daicel Pharma offers a Certificate of Analysis (CoA) for Remdesivir impurity standards that involve (R)-Tri O-benzyl nitrile Impurity, N-Butyl (SRR) Remdesivir, RDV pentafluoro RR isomer, Remdesivir Impurity- 1, Remdesivir Impurity 8, Remdesivir Impurity C, Remdesivir Impurity- 18, Remdesivir Impurity 14, Remdesivir R-P D-Alanine isomer, Remdesivir- S-Isomer at CN, and more. Our cGMP-certified analytical laboratory provides CoA with detailed characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. More characterization details, such as those for 13C-DEPT, can be provided on request. Daicel Pharma specializes in synthesizing Remdesivir impurities and degradation products.
References
FAQ's
References
- Chun, Byoung Kwon; Clarke, Michael O' Neil Hanrahan; Doerffler, Edward; Hui, Hon Chung; Jordan, Robert; Mackman, Richard L.; Parrish, Jay P.; Ray, Adrian S.; Siegel, Dustin, Methods For Treating Filoviridae Virus Infections, Gilead Sciences, Inc., United States, EP3366295B1, January 22, 2020
- Alvarez, Jean-Claude; Moine, Pierre; Etting, Isabelle; Annane, Djillali; Larabi, Islam Amine, Quantification of plasma remdesivir and its metabolite GS-441524 using liquid chromatography coupled to tandem mass spectrometry. Application to a Covid-19 treated patient, Clinical Chemistry and Laboratory Medicine, Volume: 58, Issue: 9, Pages: 1461-1468, 2020
Frequently Asked Questions
What is the potential source of Remdesivir impurities in the synthesis process?
The potential sources of Remdesivir impurities in the synthetic process include starting materials, reagents, intermediates, and degradation products.
How are Remdesivir impurities managed during the storing and distribution process?
Remdesivir impurities are managed during the storing and distribution process through strict quality control measures, proper storage conditions, and adherence to regulatory guidelines to ensure drug integrity, purity, and stability.
Are Remdesivir impurities evaluated during clinical trials?
Yes, impurities in Remdesivir are evaluated during clinical trials to ensure the safety and efficacy of the medication.
What are the temperature conditions required to store Remdesivir impurities?
Remdesivir impurities should be stored at a controlled room temperature between 2-8°C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.