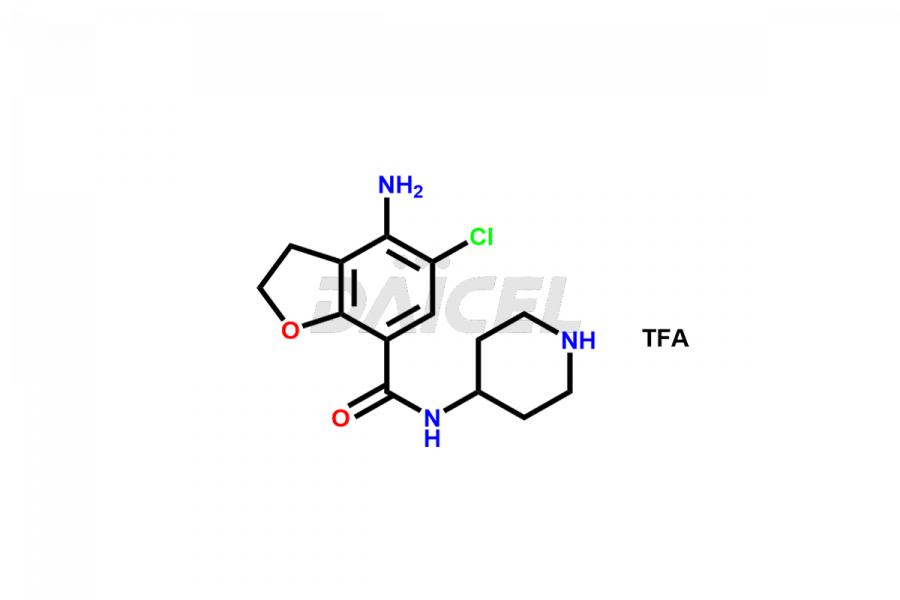
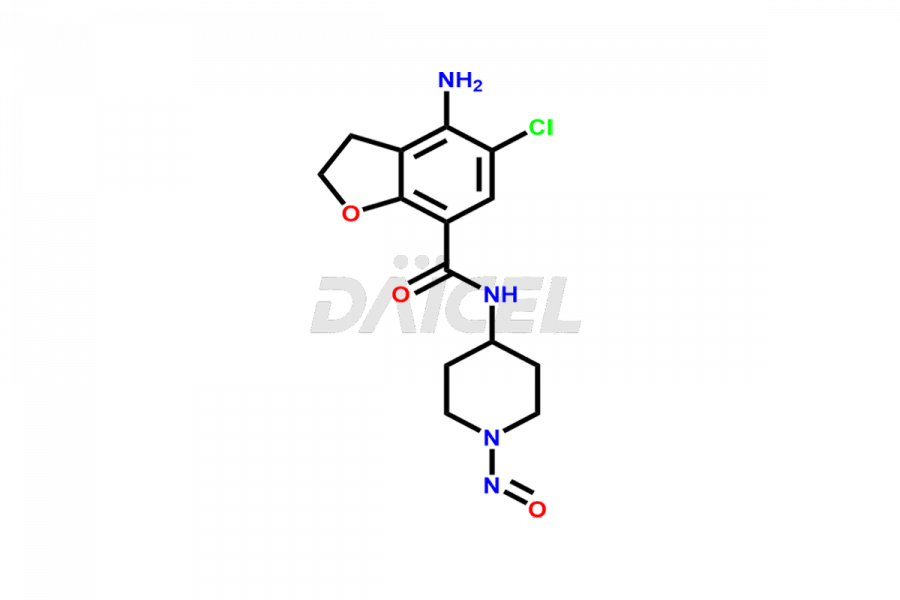
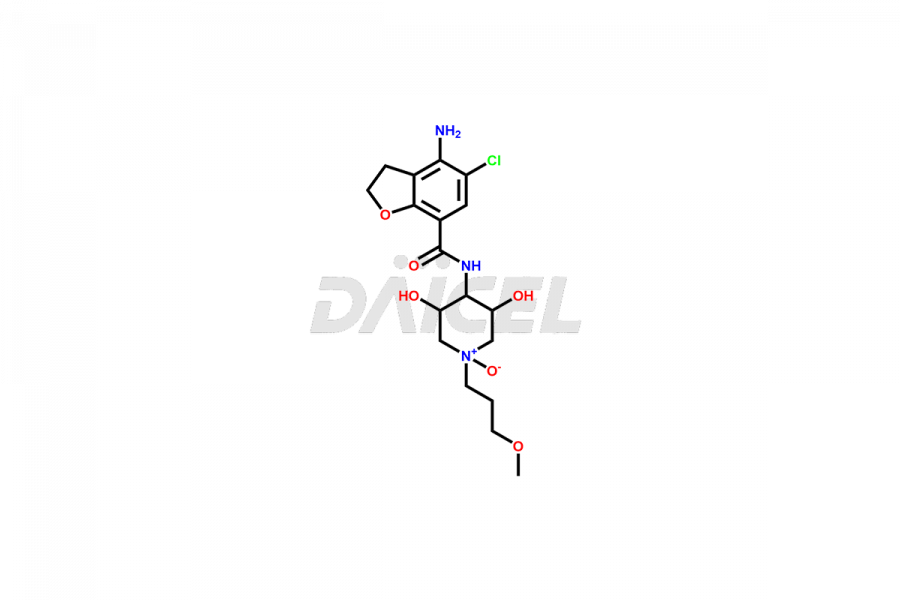
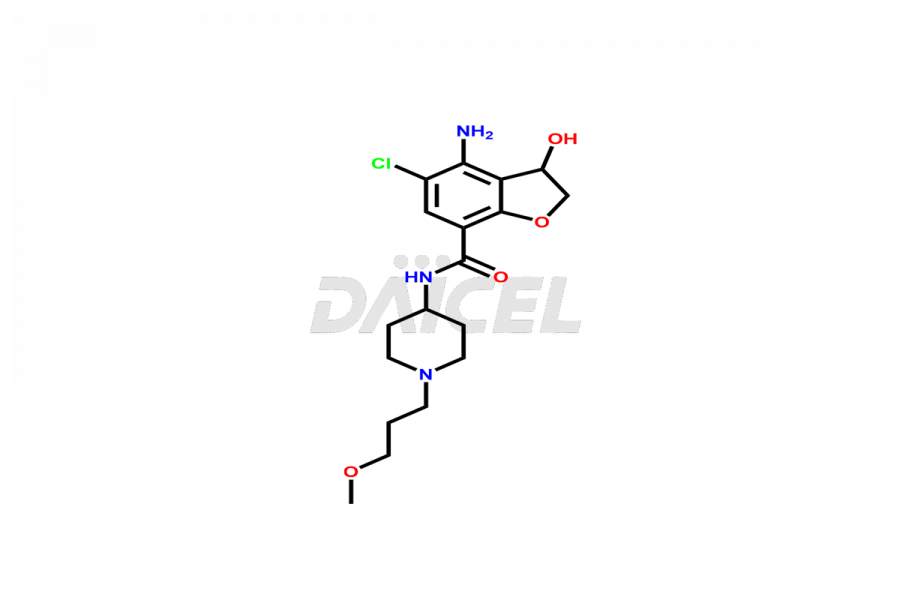
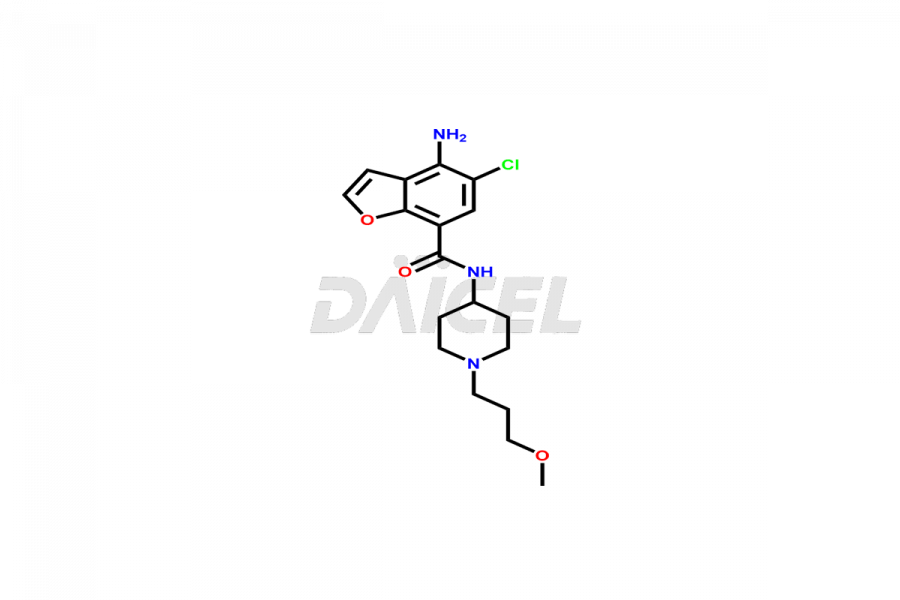
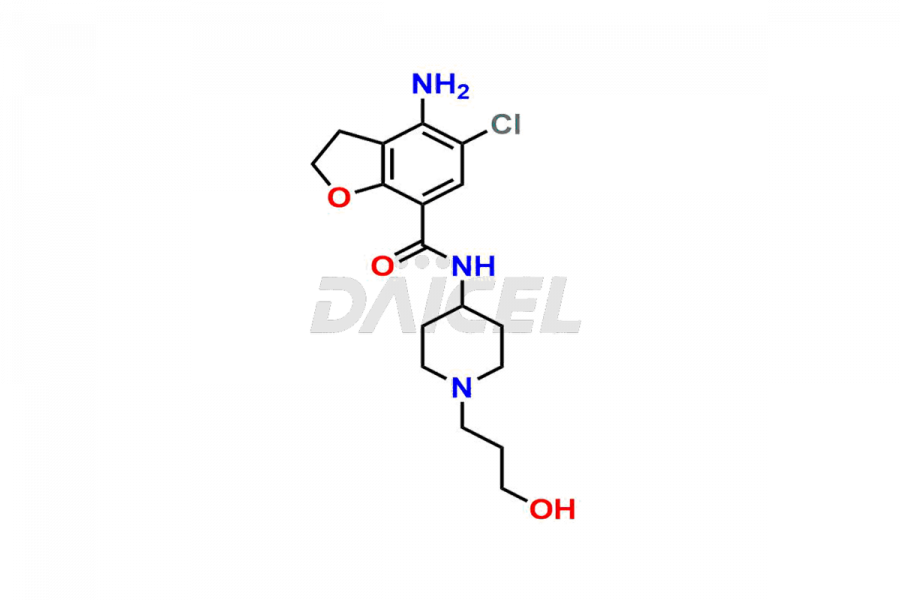
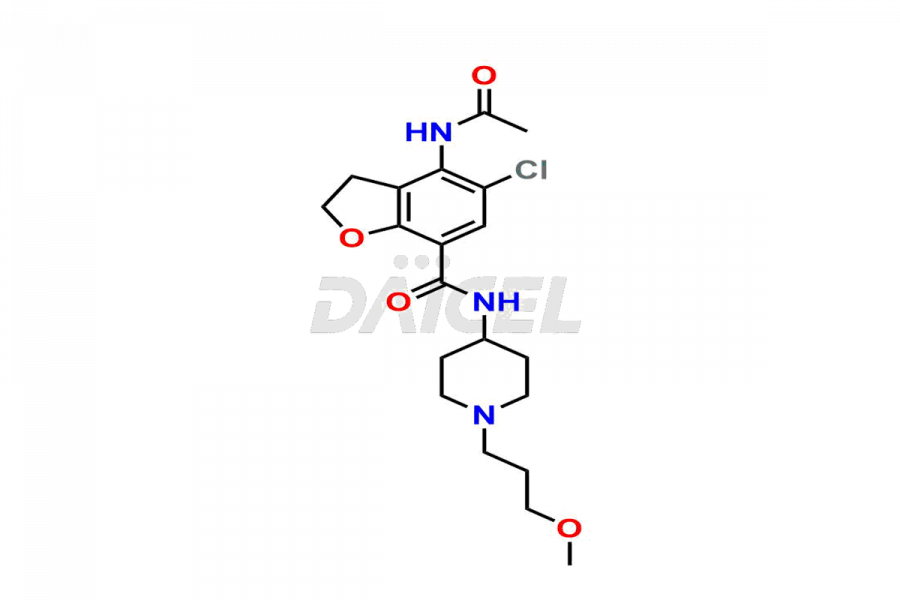
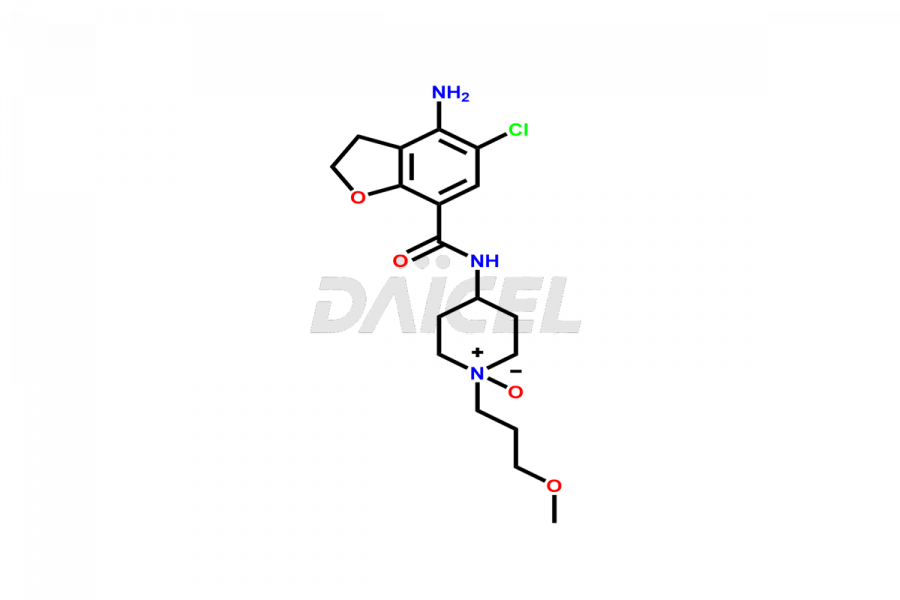
Prucalopride
General Information
Prucalopride Impurities and Prucalopride
Daicel Pharma synthesizes Prucalopride impurity standards such as Prucalopride RC 4, Prucalopride Hydroxy Impurity, Nitroso Derivative of Prucalopride Impurity-A, Prucalopride Succinate Impurity 1, Prucalopride succinate Impurity-3, Prucalopride succinate Impurity-9, Prucalopride succinate Impurity-8, N-Desmethoxypropyl Prucalopride, and more. They are crucial for the assessment of the effectiveness, stability, and safety of Prucalopride. Daicel Pharma provides a custom synthesis for Prucalopride impurities, ensuring worldwide delivery to cater to the unique requirements of our clients.
Prucalopride [CAS: 179474-81-8] is a Serotonin type-4 Receptor agonist for treating constipation. It is a dihydrobenzofurancarboxamide derivative for the symptomatic treatment of chronic constipation.
Prucalopride: Use and Commercial Availability
Prucalopride is for adults suffering from chronic idiopathic constipation (CIC), among the most prevalent chronic functional gastrointestinal disorders globally. This drug is available under the brand name Motegrity.
Prucalopride Structure and Mechanism of Action
The chemical name of Prucalopride is 4-Amino-5-chloro-2,3-dihydro-N-[1-(3-methoxypropyl)-4-piperidinyl]-7-benzofurancarboxamide. Its chemical formula is C18H26ClN3O3, and its molecular weight is approximately 367.9 g/mol.
Prucalopride stimulates colonic peristalsis and increases bowel motility.
Prucalopride Impurities and Synthesis
Impurities in Prucalopride can arise either during manufacturing1 or degradation over time. Their presence can compromise the drug quality, effectiveness, and safety. Consequently, in-depth analysis and regulation of Prucalopride impurities are indispensable to fulfill regulatory standards and safety requirements.
Daicel Pharma offers a Certificate of Analysis (CoA) for Prucalopride impurity standards, Prucalopride RC 4, Prucalopride Hydroxy Impurity, Nitroso Derivative of Prucalopride Impurity-A, Prucalopride Succinate Impurity 1, Prucalopride succinate Impurity-3, Prucalopride succinate Impurity-9, Prucalopride succinate Impurity-8, N-Desmethoxypropyl Prucalopride, and more. The Certificate of Analysis (CoA) contains a detailed characterization report with information from techniques, including 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. Additionally, upon request, we can supply supplementary data such as 13C-DEPT. Further, upon request, Daicel Pharma can offer unknown Prucalopride impurities and labeled compounds to assess the effectiveness of Prucalopride.
References
FAQ's
References
- Van Daele, Georges Henri Paul; Bosmans, Jean-Paul Rene Marie Andre; Schuurkes, Joannes Adrianus Jacobus, Enterokinetic Benzamide, Janssen Pharmaceutica N.V., Belgium, EP807110B1, May 8, 2002
- Vaibhavi N. Akhani, Mrs. Khushbu K. Patel, Ms. K. S. Patel, Dr. L.M. Prajapati and Dr. C. N. Patel, Development And Validation Of RP-HPLC Method For Estimation Of Prucalopride Succinate In Pharmaceutical Dosage Form, World Journal Of Pharmacy And Pharmaceutical Sciences, Volume 9, Issue 6, 1112-1122, 06 May 2020 DOI: 10.20959/wjpps20206-16234
Frequently Asked Questions
What are the permissible levels of Prucalopride impurities in pharmaceutical products?
The permissible levels of Prucalopride impurities in pharmaceutical products vary depending on regulatory guidelines and specific requirements. It is essential to comply with the established limits set by regulatory authorities to ensure the safety and quality of the drug.
How are Prucalopride impurities regulated throughout the production process?
Impurities in Prucalopride are regulated throughout the production process by implementing stringent quality control measures, rigorous analytical testing, and adherence to regulatory guidelines to ensure that impurity levels are within acceptable limits, thereby maintaining the safety and efficacy of the drug.
Is it possible that the presence of specific impurities may influence the stability of Prucalopride?
The presence of impurities in Prucalopride can impact its stability due to potential interactions or chemical reactions between the impurities and the active pharmaceutical ingredient.
What are the temperature conditions required to store Prucalopride impurities?
Prucalopride impurities are stored preferably at a regulated room temperature of 2-8°C or as specified on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.