General Information
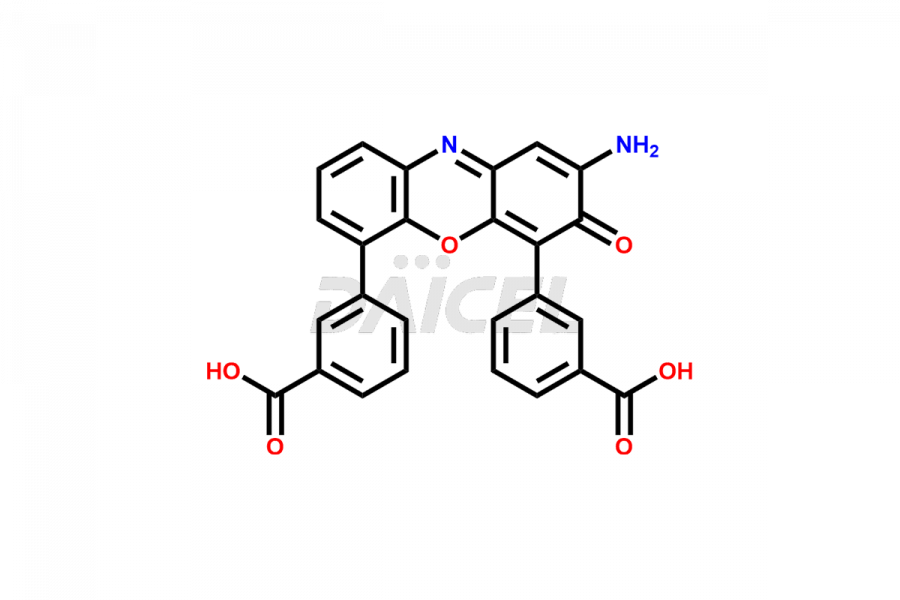
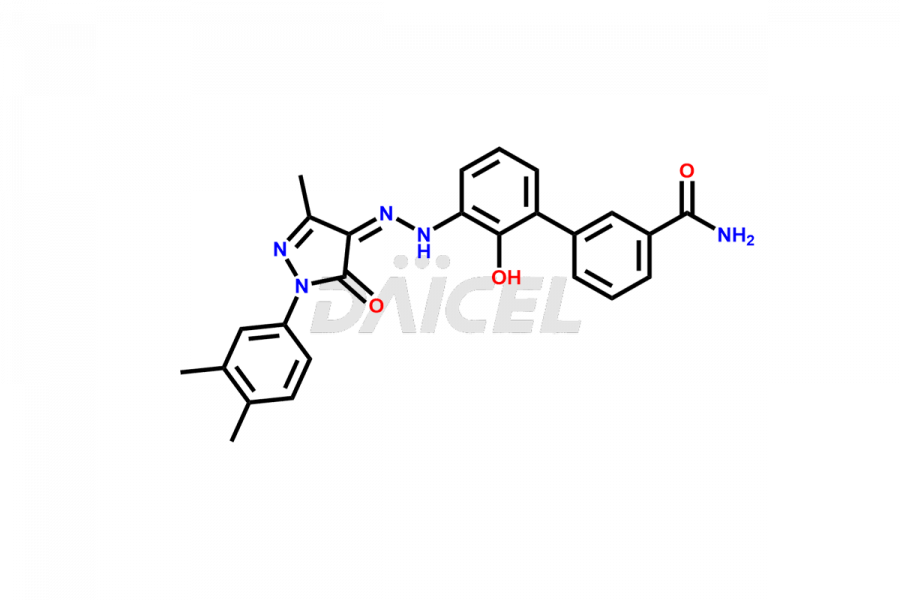
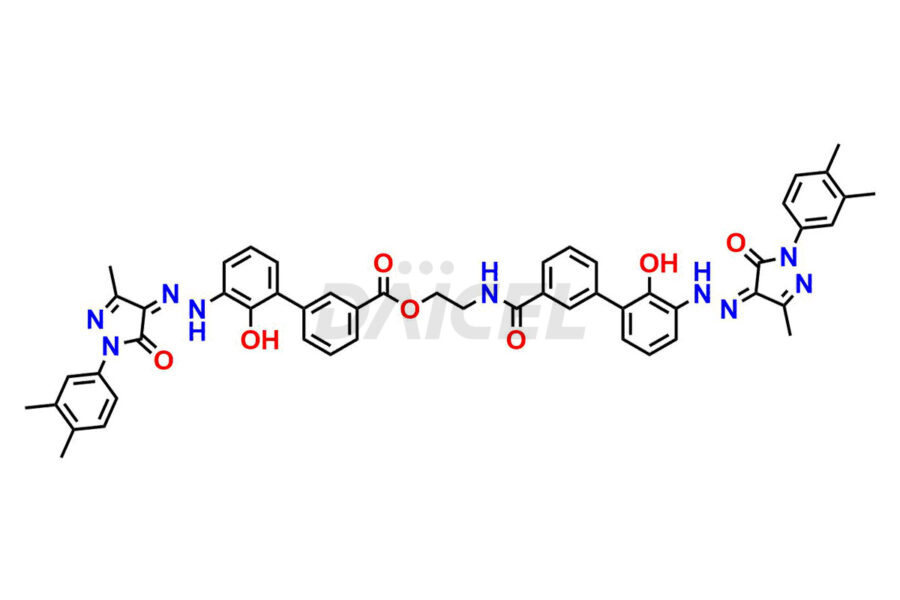
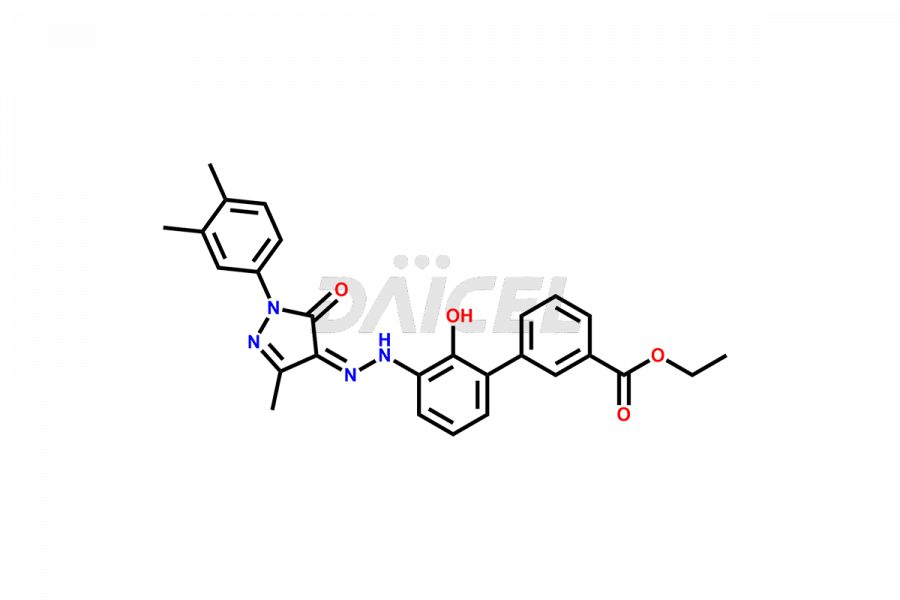
Eltrombopag Impurities and Eltrombopag
Daicel Pharma offers high-quality impurities for Eltrombopag, an active pharmaceutical ingredient. These impurities, including Eltrombopag amide impurity and Eltrombopag dimer-1 impurity, play a vital role in assessing Eltrombopag purity, reliability, and safety. Daicel Pharma also offers a customized synthesis of Eltrombopag impurities to cater to client requirements, with worldwide delivery options available.
Eltrombopag [CAS: 496775-61-2], a medication to treat thrombocytopenia, acts as an orally active agonist for the thrombopoietin receptor, stimulating megakaryopoiesis (the production of platelets).
Eltrombopag: Use and Commercial Availability
Eltrombopag, available under brand names such as Alvaiz, Promacta Kit, and Promacta, is a small molecule administered orally. It acts as a non-peptide agonist of thrombopoietin receptors, effectively increasing platelet counts. It enhances the proliferation and differentiation of progenitor cells in the bone marrow by activating intracellular signal transduction pathways. Eltrombopag treat chronic idiopathic thrombocytopenic purpura (ITP), an autoimmune disease. These drugs stimulate the thrombopoietin receptor to promote platelet production and reduce the risk of bleeding complications. They help patients who cannot undergo other treatment options, such as immunoglobulins, corticosteroids, or splenectomy.
Eltrombopag Structure and Mechanism of Action 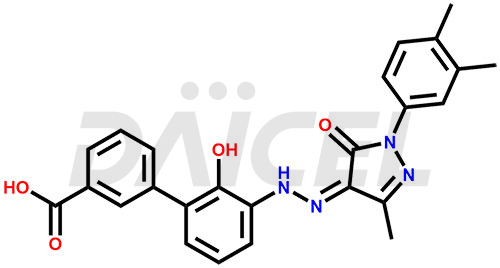
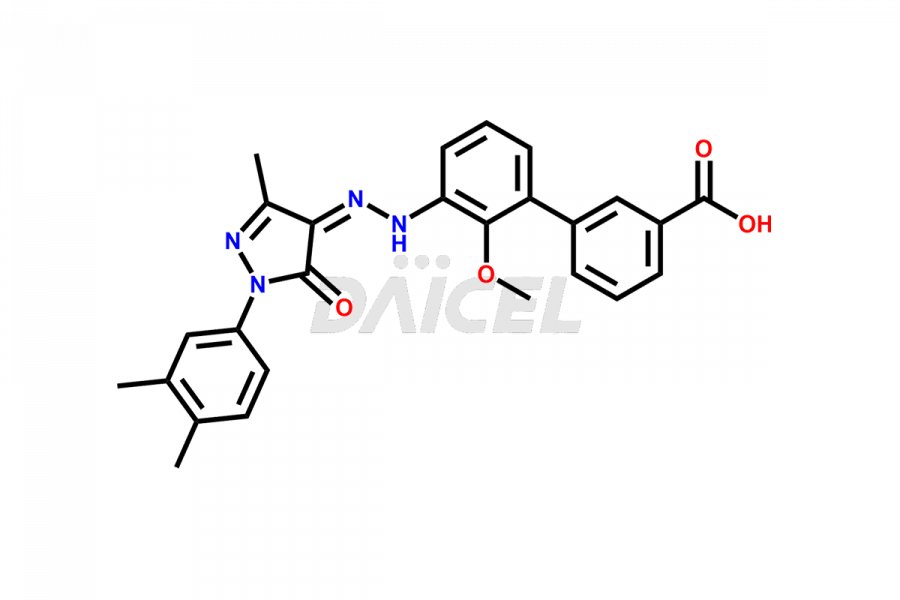
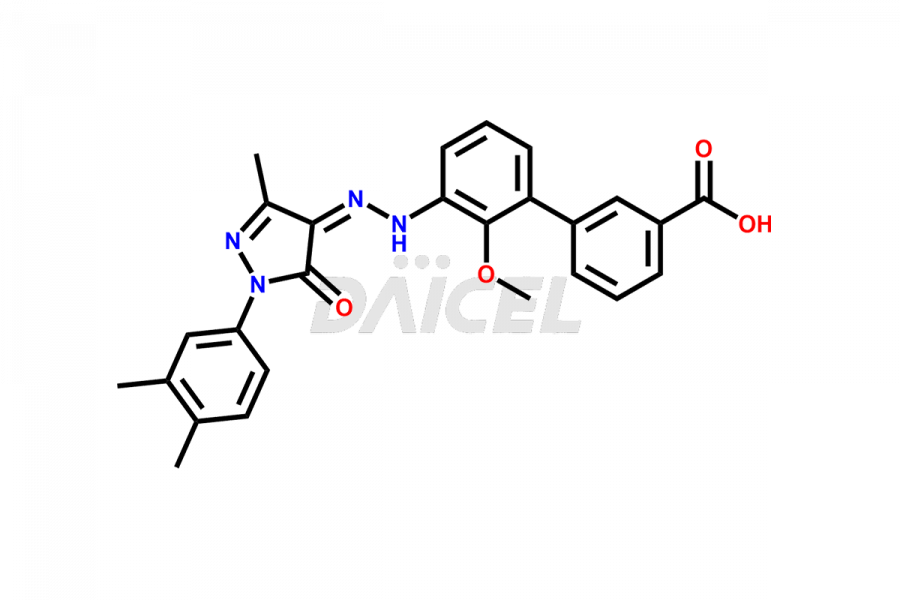
The chemical name of Eltrombopag is 3′-[(2Z)-2-[1-(3,4-Dimethylphenyl)-1,5-dihydro-3-methyl-5-oxo-4H-pyrazol-4-ylidene]hydrazinyl]-2′-hydroxy[1,1′-biphenyl]-3-carboxylic acid. Its chemical formula is C25H22N4O4, and its molecular weight is approximately 442.5 g/mol.
Eltrombopag binds and stimulates the platelet thrombopoietin receptor (TPO-R), leading to the proliferation and differentiation of megakaryocytes, resulting in increased production of blood platelets.
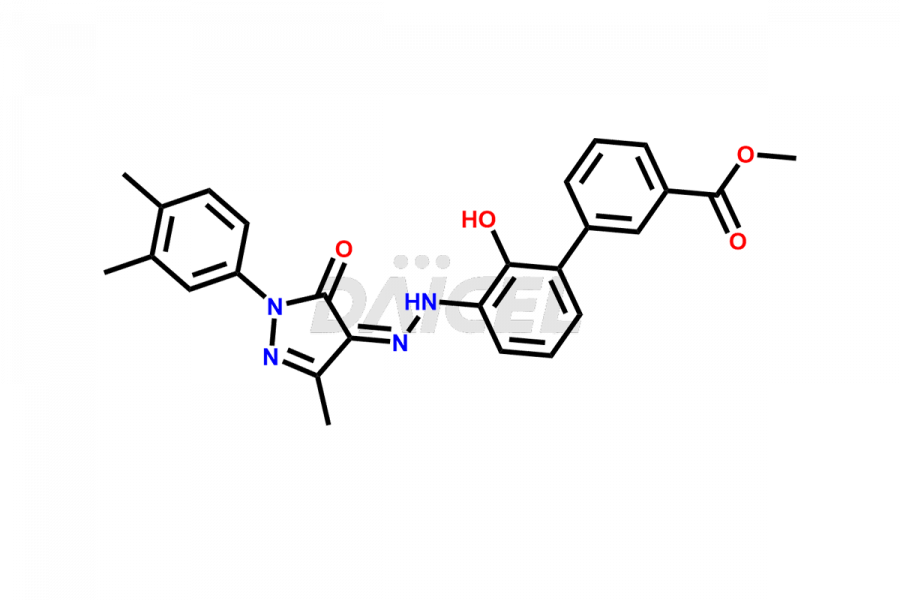
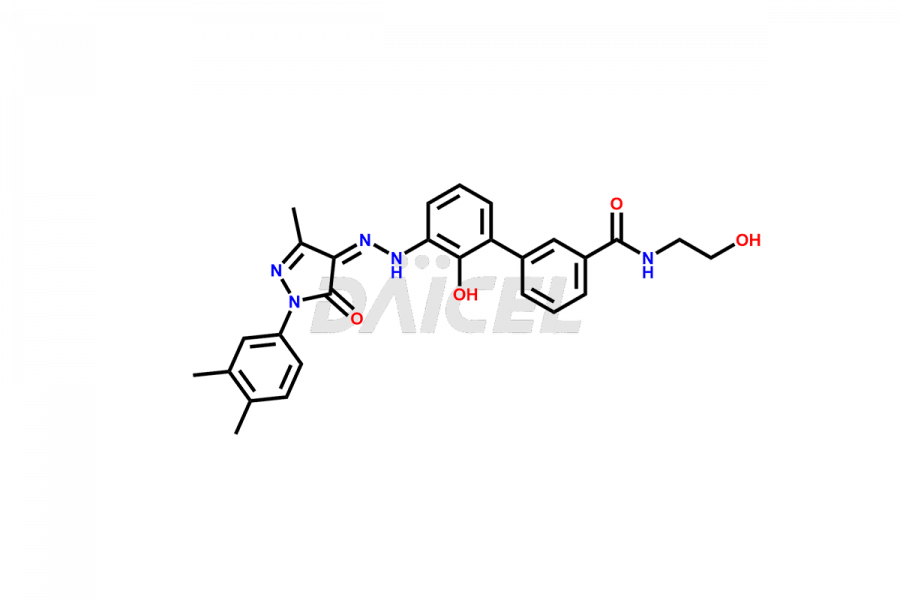
Eltrombopag Impurities and Synthesis
Accurate analysis and effective control of impurities in Eltrombopag, a medication used to increase platelet counts, are crucial to ensure its safety and efficacy. Analytical techniques such as chromatography, spectroscopy, and mass spectrometry help identify and quantify impurities in Eltrombopag. Impurity profiling aids in understanding the chemical composition, structure, and potential risks associated with drug impurities. Stringent control measures during the manufacturing process1 help minimize impurity formation. Regulatory guidelines define acceptable limits for Eltrombopag impurities in pharmaceutical products, and thorough analysis and control are vital for upholding their quality and therapeutic effectiveness for patients.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Eltrombopag impurity standards, including Eltrombopag amide impurity and Eltrombopag dimer-1 impurity. They generate from an analytical facility that complies with cGMP standards. The CoA provides a detailed characterization report with data obtained through techniques such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis2. We provide additional data like 13C-DEPT upon request. Daicel Pharma synthesizes unknown Eltrombopag impurities or degradation products. Every delivery has a complete characterization report.