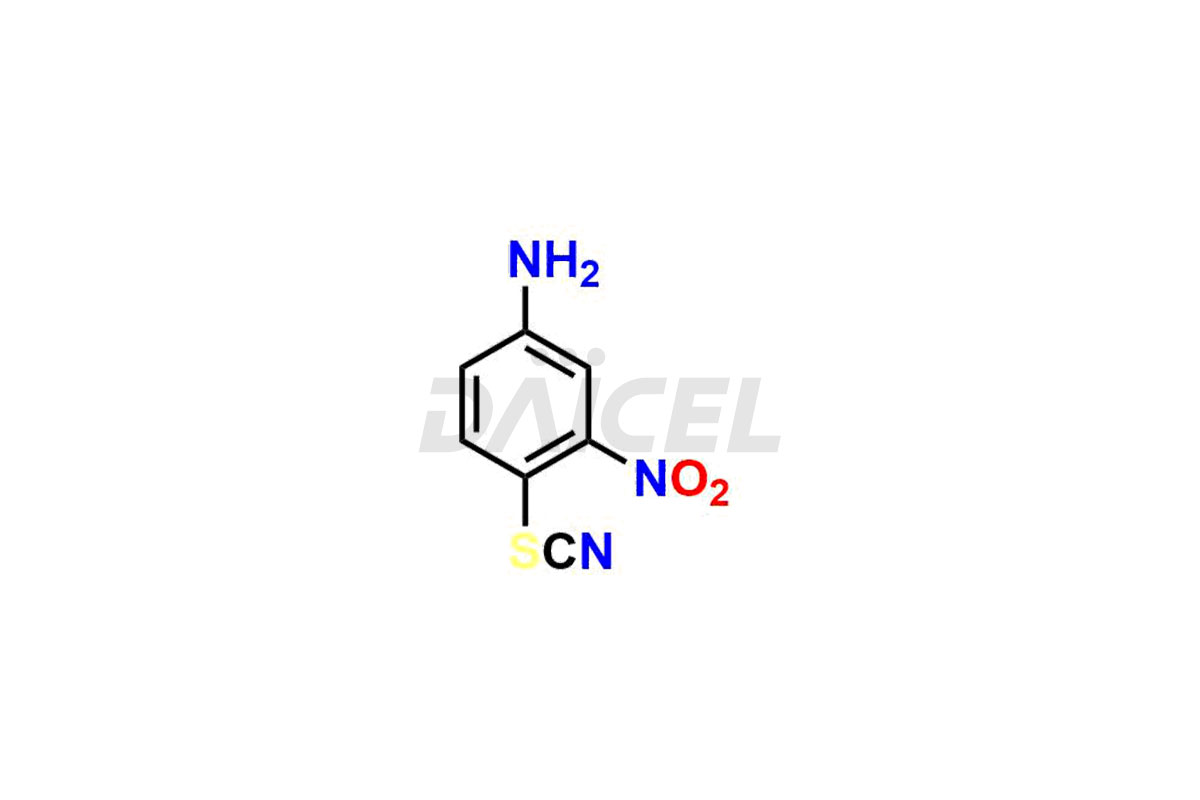
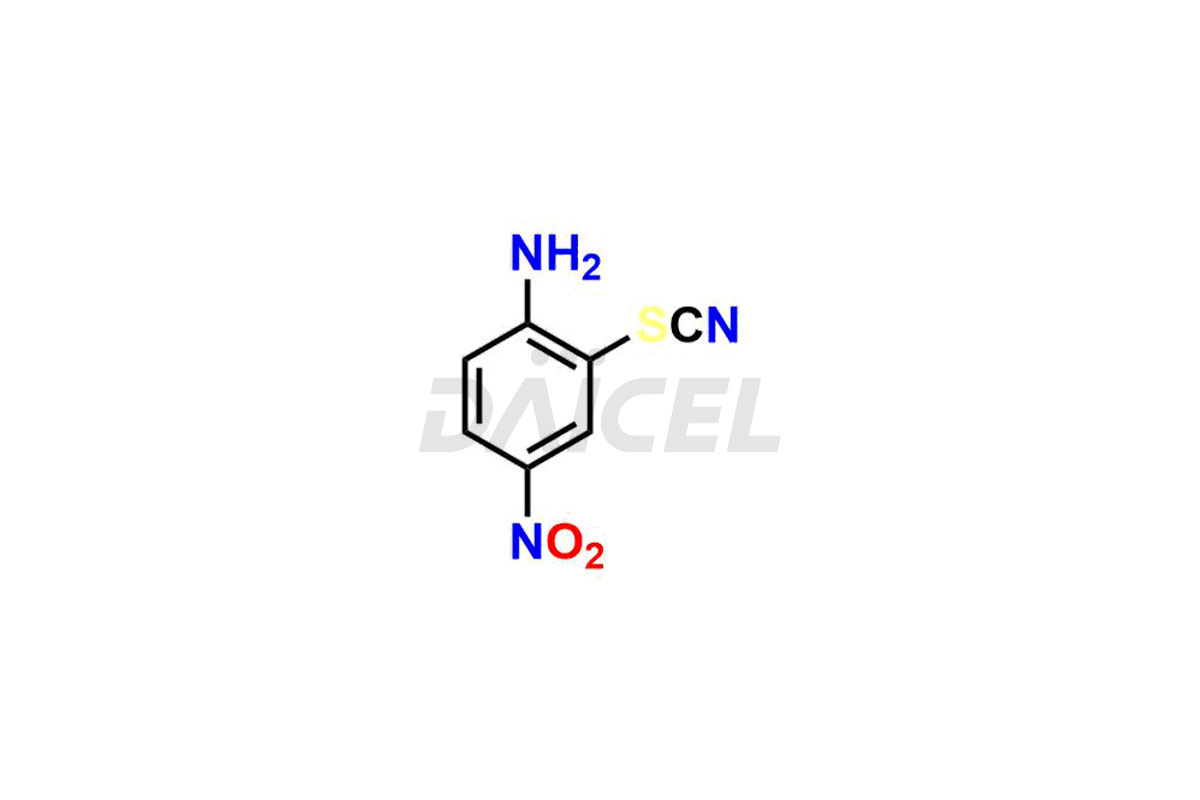
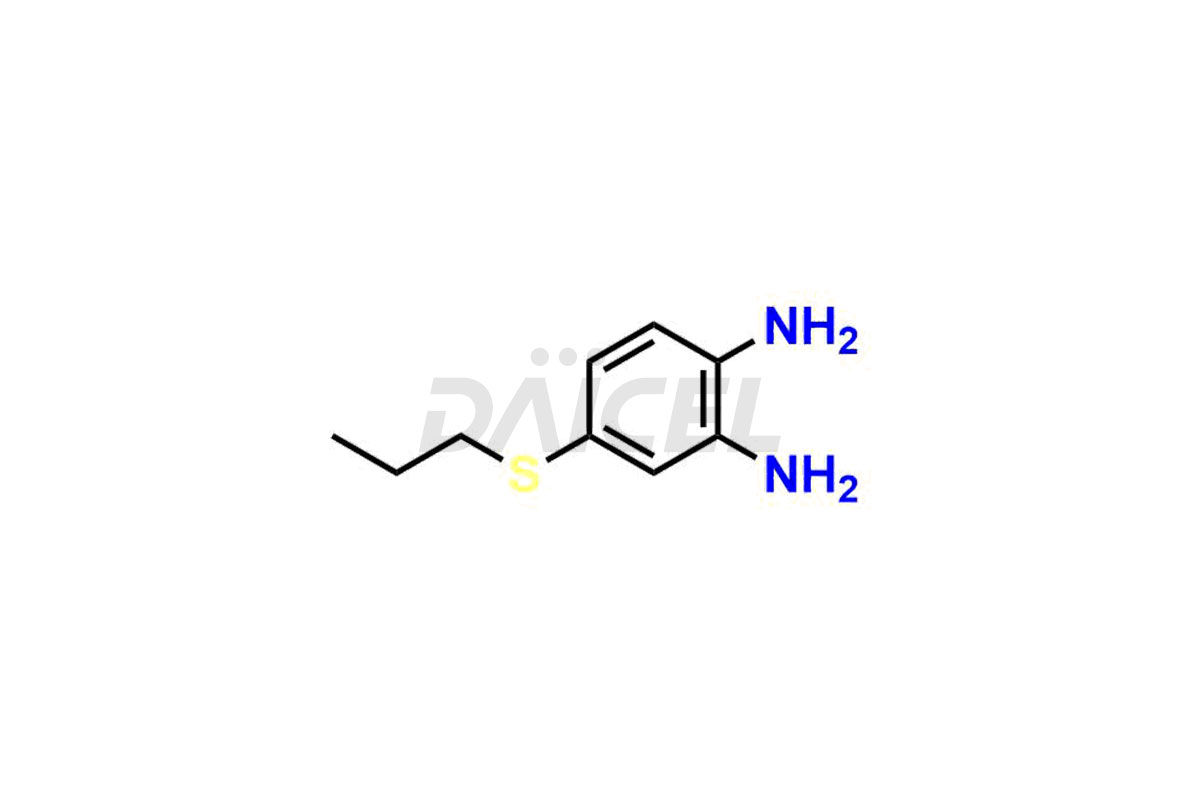
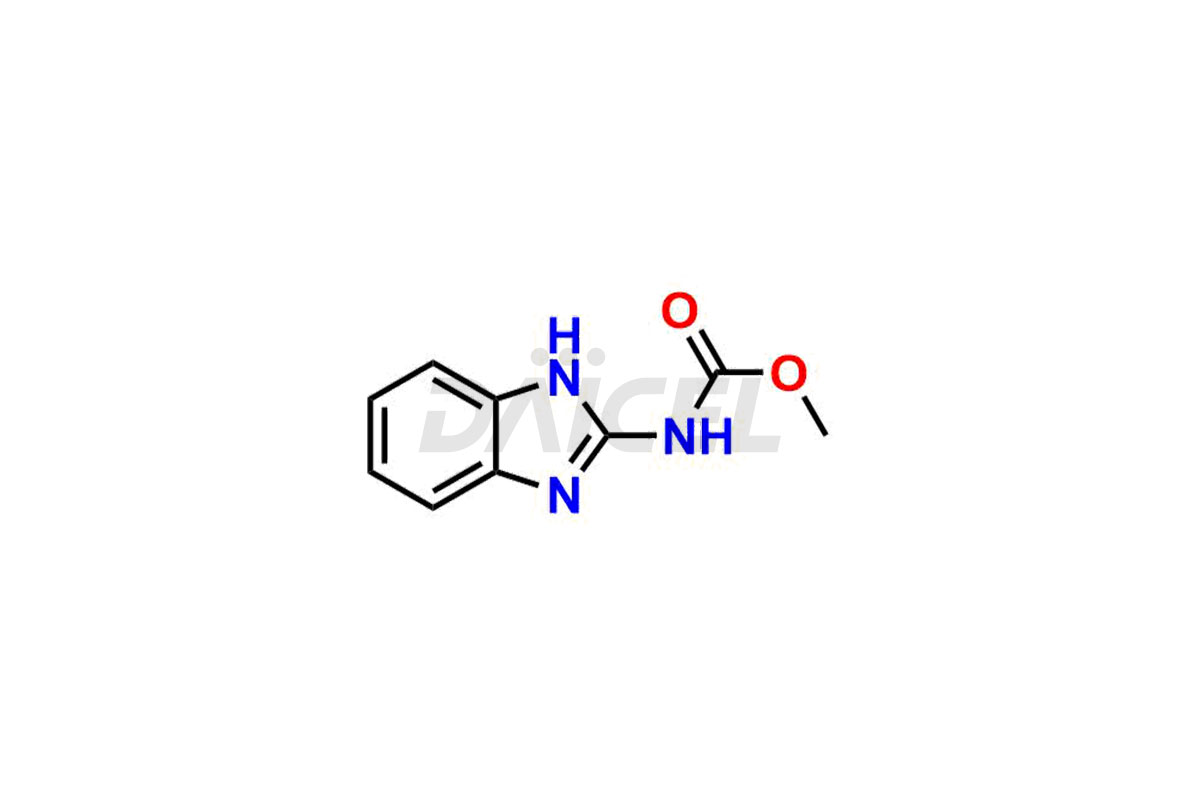
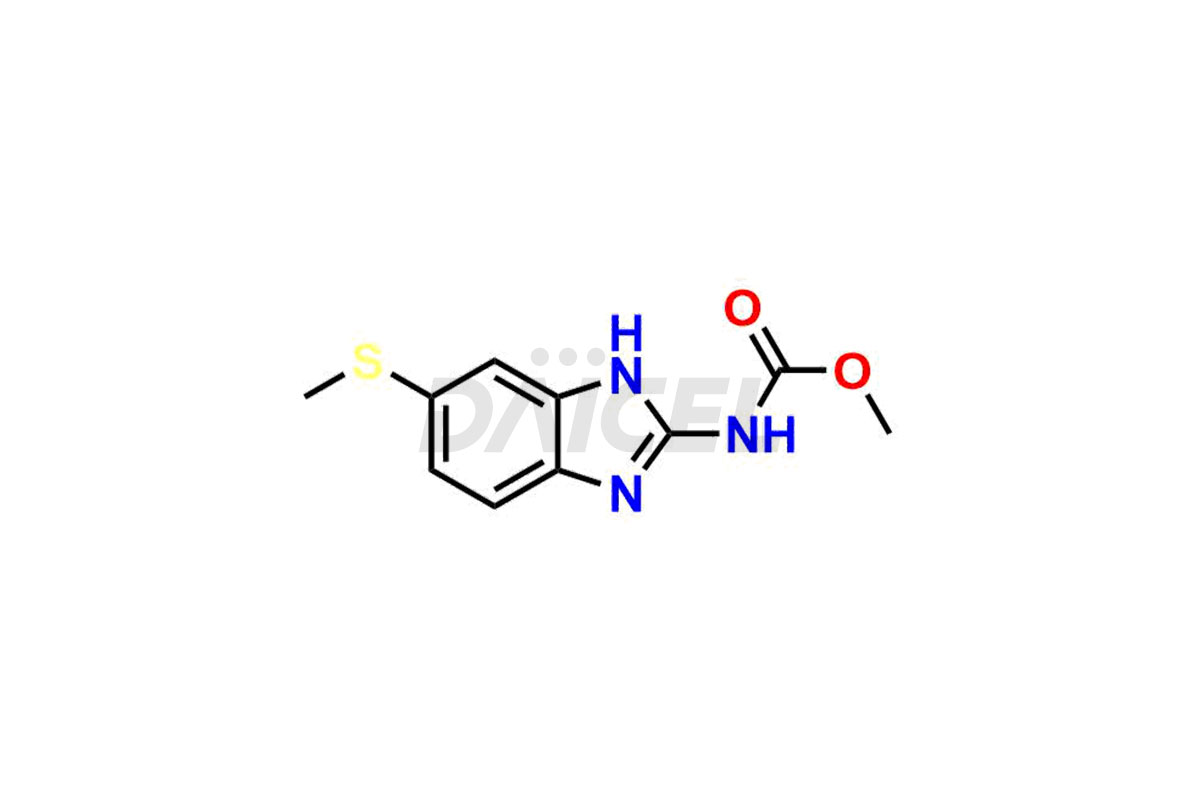
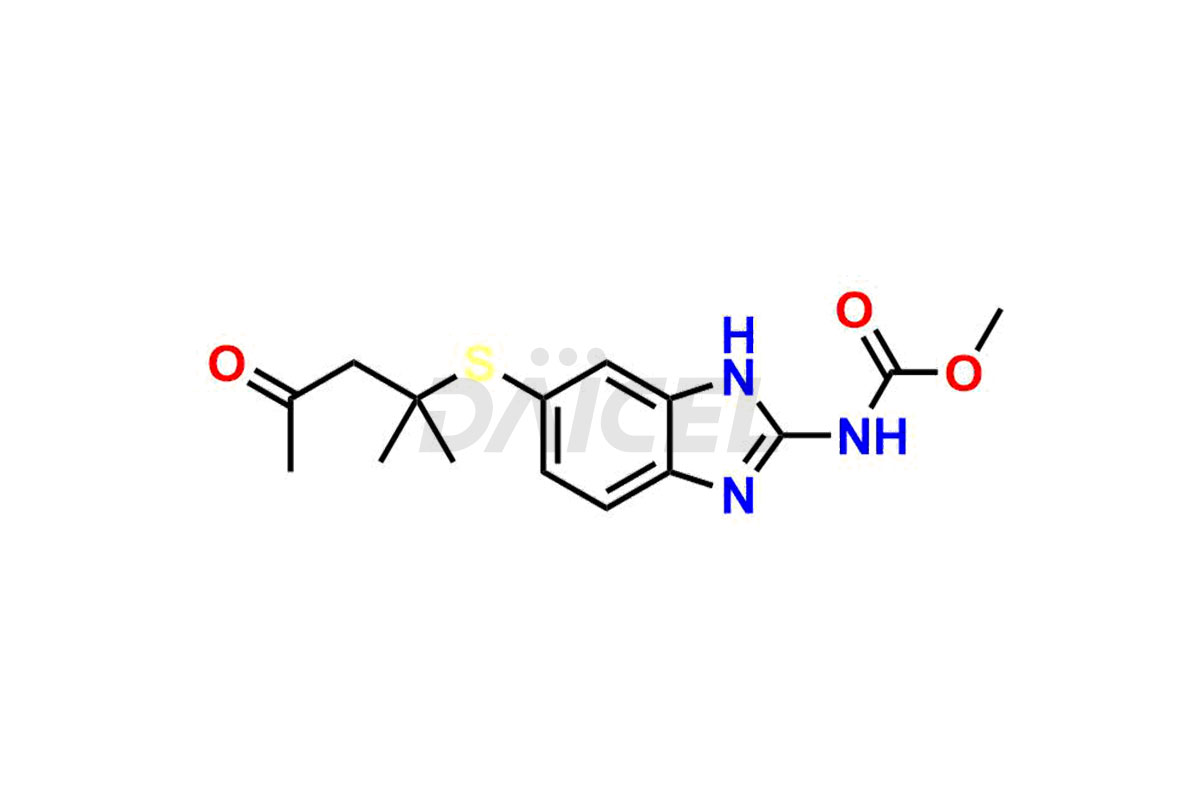
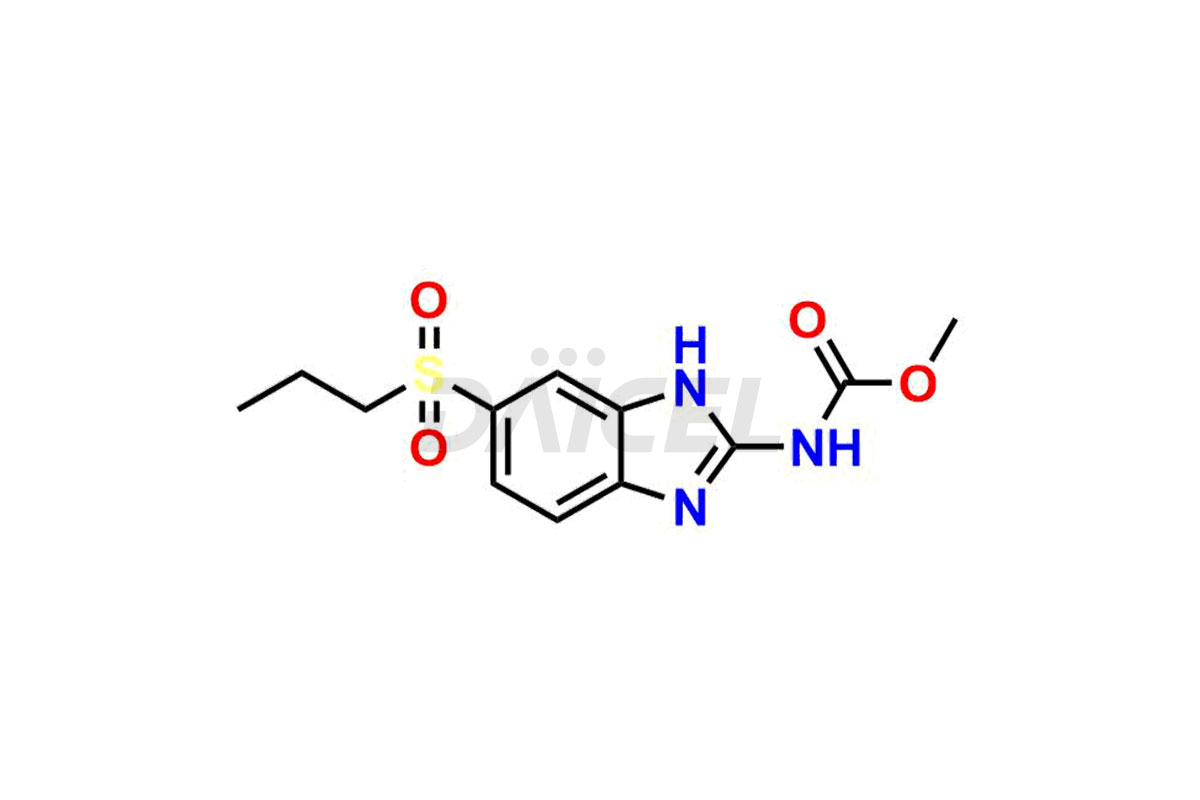
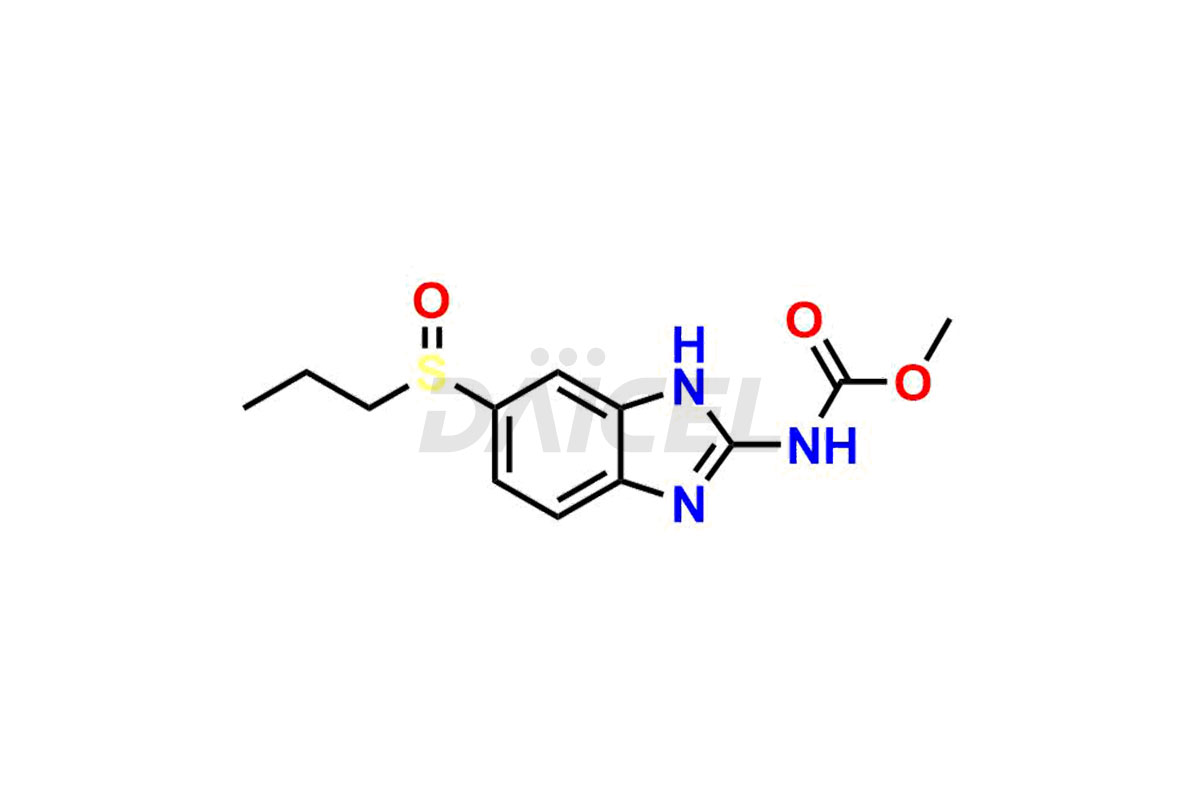
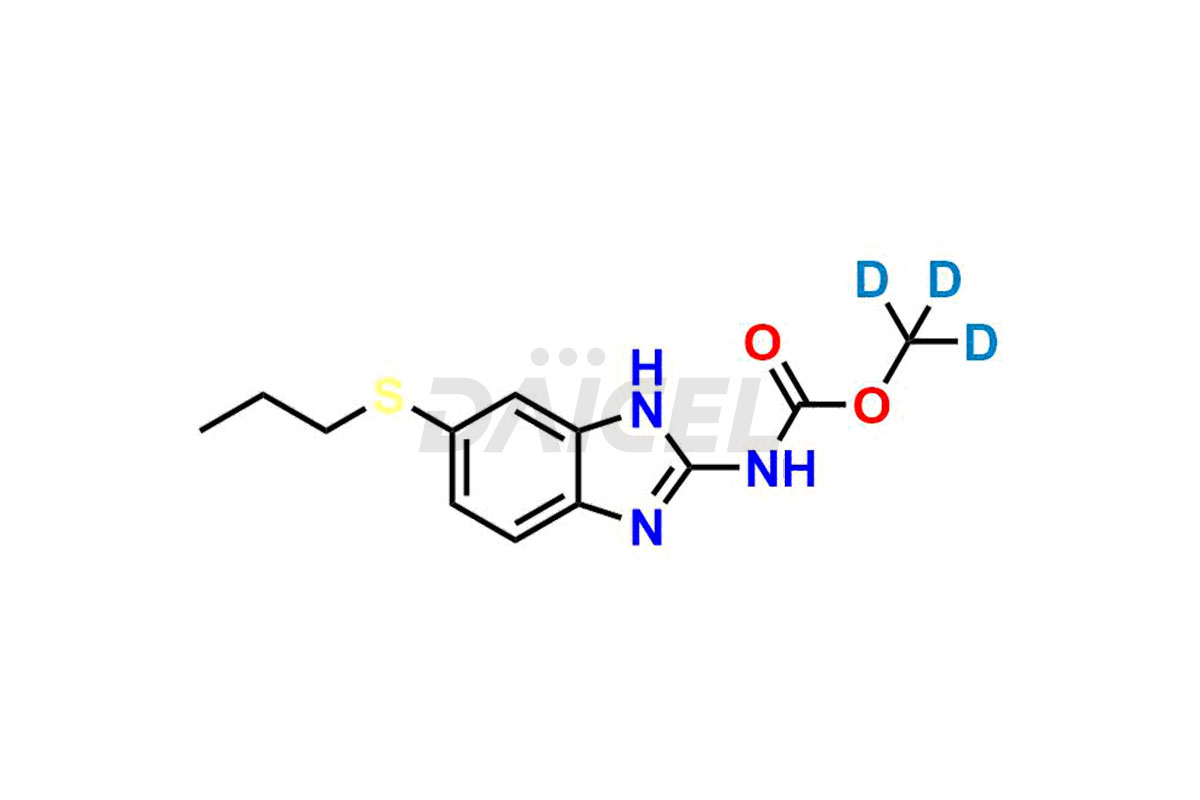
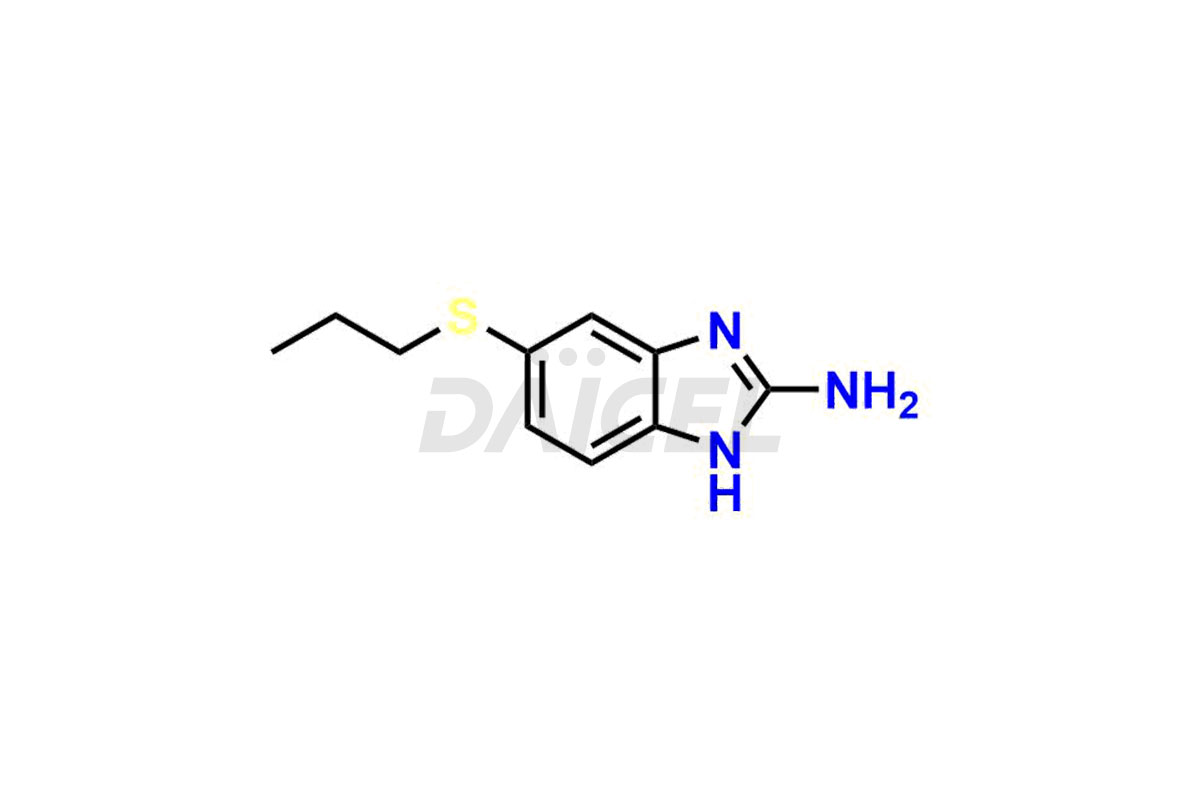
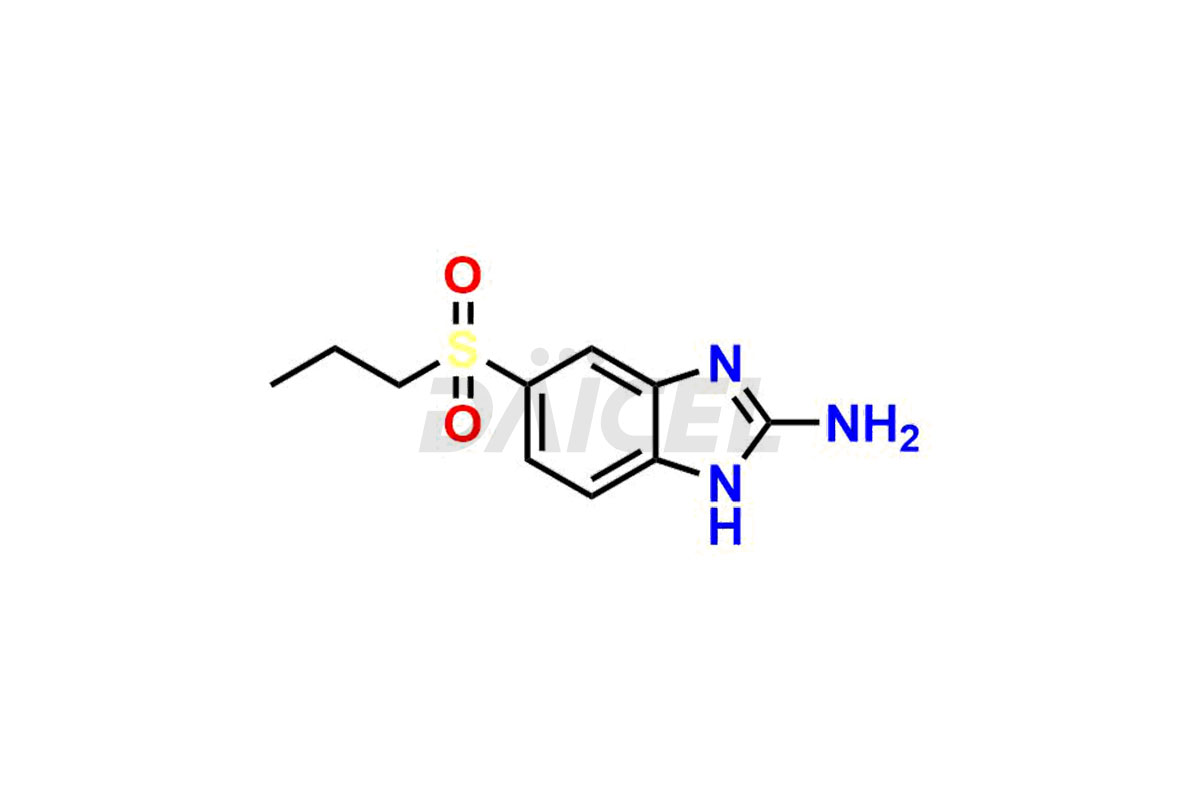
Albendazole
General Information
Albendazole Impurities and Albendazole
Daicel Pharma is a reliable source for synthesizing high-quality Albendazole impurities, specifically Albendazole impurity (Albendazole EP Impurity-H), Albendazole sulfoxide (Albendazole EP impurity B), Albendazole sulfone (Albendazole EP impurity C), and more. These impurities help assess the quality, stability, and safety of the active pharmaceutical ingredient, Albendazole. Daicel Pharma also offers custom synthesis of Albendazole impurities, which can be shipped worldwide to meet customers’ unique requirements.
Albendazole [CAS: 54965-21-8] is a broad-spectrum drug. It is a synthetic benzimidazole-derivative anthelmintic, which can kill many parasitic worms in the body. It treats echinococcosis, a parasitic worm that forms cysts in the liver and lungs.
Albendazole: Use and Commercial Availability
Albendazole is an antiparasitic medication approved by the US FDA for treating specific parasitic worm infections. It treats microsporidiosis, which can be an opportunistic infection (OI) of HIV. Albendazole is available under various brand names. As an antihelminthic medication, Albendazole can treat several parasitic worm infections. It treats cystic hydatid disease in the liver, lungs, and peritoneum caused by Echinococcus granulosus. It also treats neurocysticercosis caused by larval forms of the pork tapeworm, Taenia solium.
Albendazole Structure and Mechanism of Action 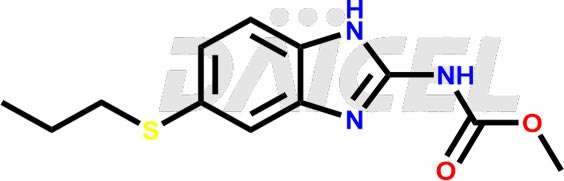
The chemical name of Albendazole is Methyl 5-propylthio-2-benzimidazolecarbamate. Its chemical formula is C12H15N3O2S, and its molecular weight is approximately 265.33 g/mol.
The anthelmintic activity of Albendazole is due to its metabolite, albendazole sulfoxide. It inhibits the formation of microtubules from tubulin, thus interfering with the reproduction and survival of helminths.
Albendazole Impurities and Synthesis
During the manufacturing process of Albendazole, impurities may form that can potentially reduce its efficacy or cause harm to patients. These impurities can arise from various sources, including starting materials, intermediates, and reagents used in the synthesis1 of Albendazole. Therefore, it is essential to control and monitor impurities to ensure the safety and effectiveness of the drug.
Daicel Pharma provides a Certificate of Analysis (CoA) for Albendazole impurity standards, which includes Albendazole impurity (Albendazole EP Impurity-H), Albendazole sulfoxide (Albendazole EP impurity B), Albendazole sulfone (Albendazole EP impurity C), and more. The CoA is from an analytical facility that adheres to current Good Manufacturing Practices (cGMP) and includes complete characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We give additional characterization data such as 13C-DEPT and CHN on request. Daicel Pharma can also generate unknown Albendazole impurities or degradation products and provide labeled compounds to assess the effectiveness of Albendazole. Further, Daicel Pharma offers Albendazole-D3 (methoxy-D3), a deuterium-labeled Albendazole compound used in bio-analytical research, such as BA/BE studies. A complete characterization report is a part of each delivery.
References
FAQ's
References
- Gyurik, Robert J.; Theodorides, Vassilios J., Methyl 5-propylthio-2-benzimidazolecarbamate, Smithkline Corp., United States, US3915986A, October 28, 1975
- Alvinerie, M.; Galtier, P., Simultaneous determination of albendazole and its principal metabolites in plasma by normal phase high-performance liquid chromatography, Journal of Pharmaceutical and Biomedical Analysis, Volume: 2, Issue: 1, Pages: 73-9, 1984
Frequently Asked Questions
What are the challenges in the analysis of Albendazole impurities?
Challenges faced during Albendazole impurity analysis include the sensitivity of analytical methods and the potential variability of impurity levels between batches.
What is the difference between process-related and product-related impurities in Albendazole?
Process-related impurities in Albendazole arise during manufacturing, while degradation impurities occur during the storage or handling of the drug product.
How are Albendazole impurities controlled during the manufacturing of the drug?
Impurities in Albendazole are controlled during manufacturing with process optimization, careful selection of raw materials, and appropriate analytical testing throughout the production process.
What are the temperature conditions required to store Albendazole impurities?
Albendazole impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.