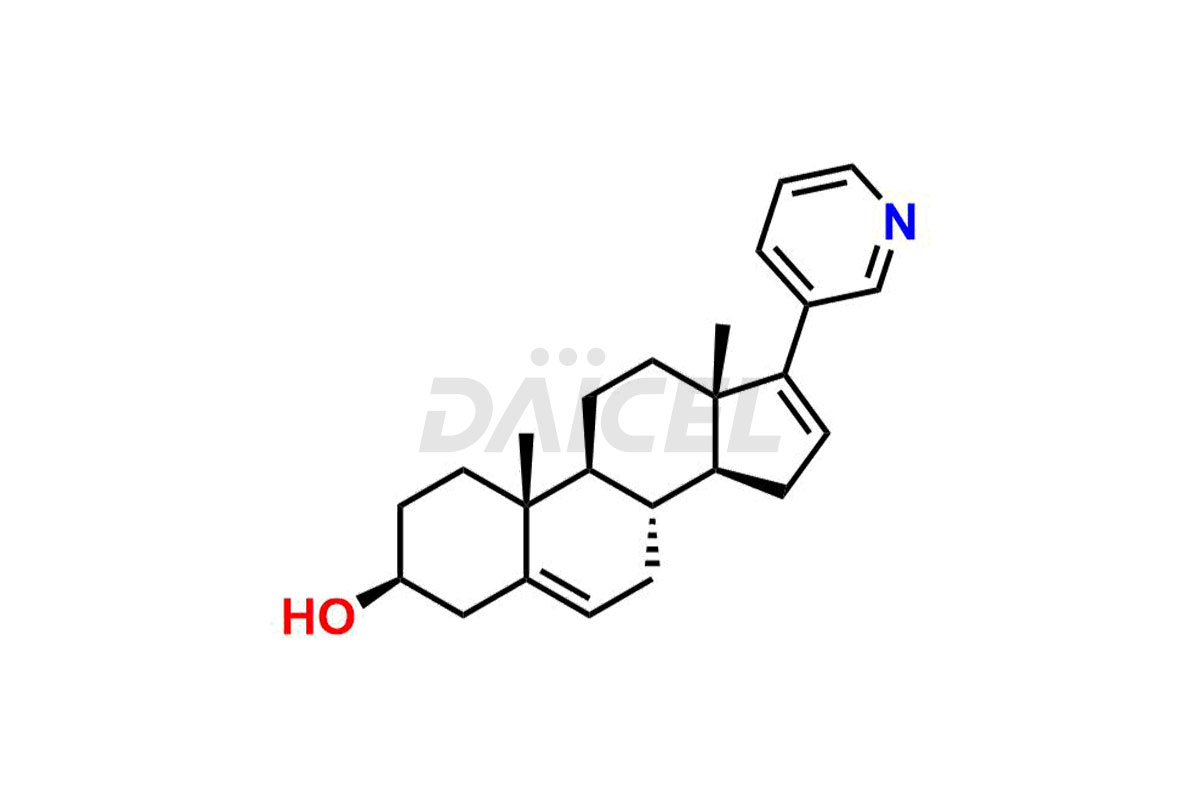
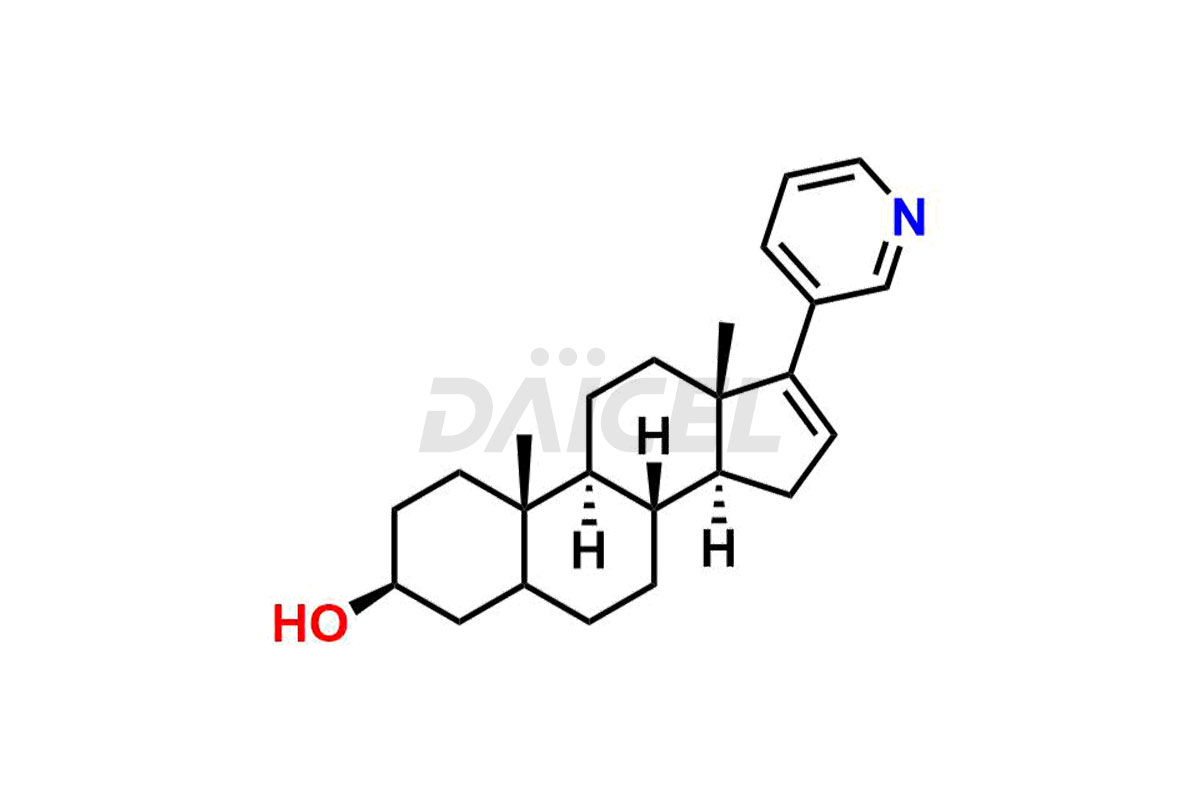
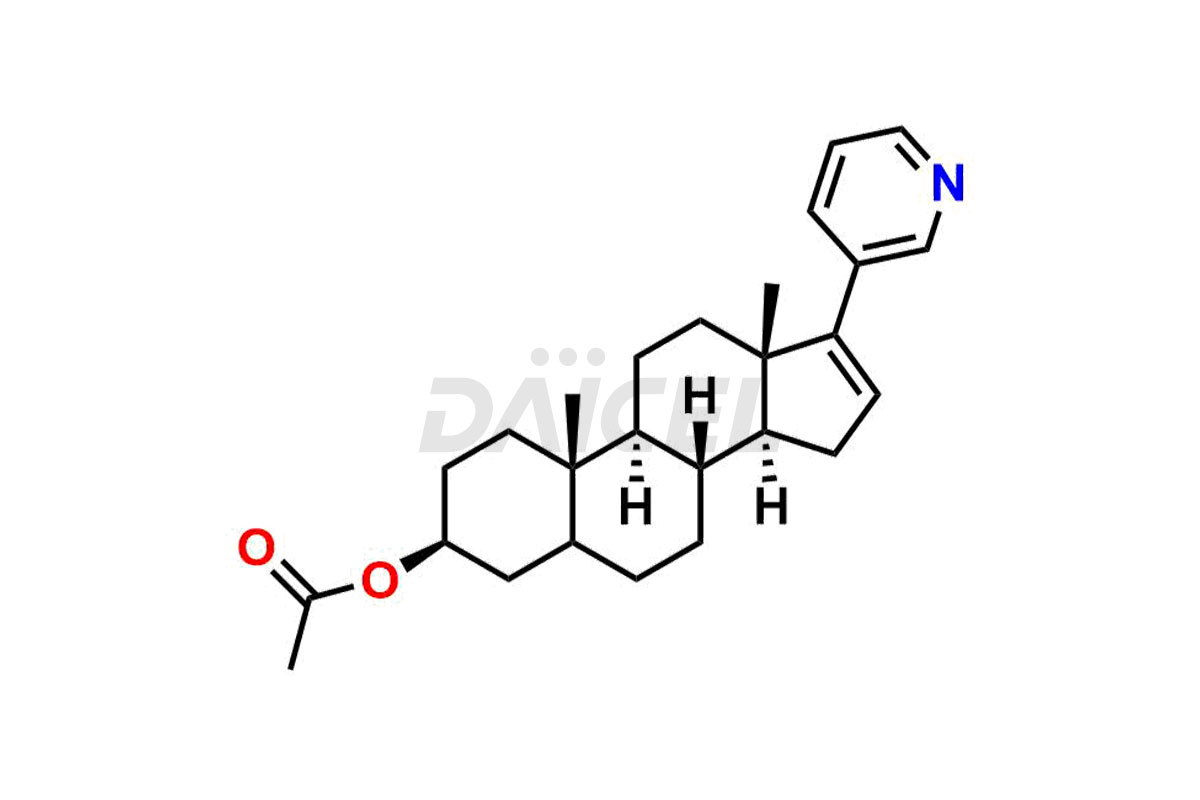
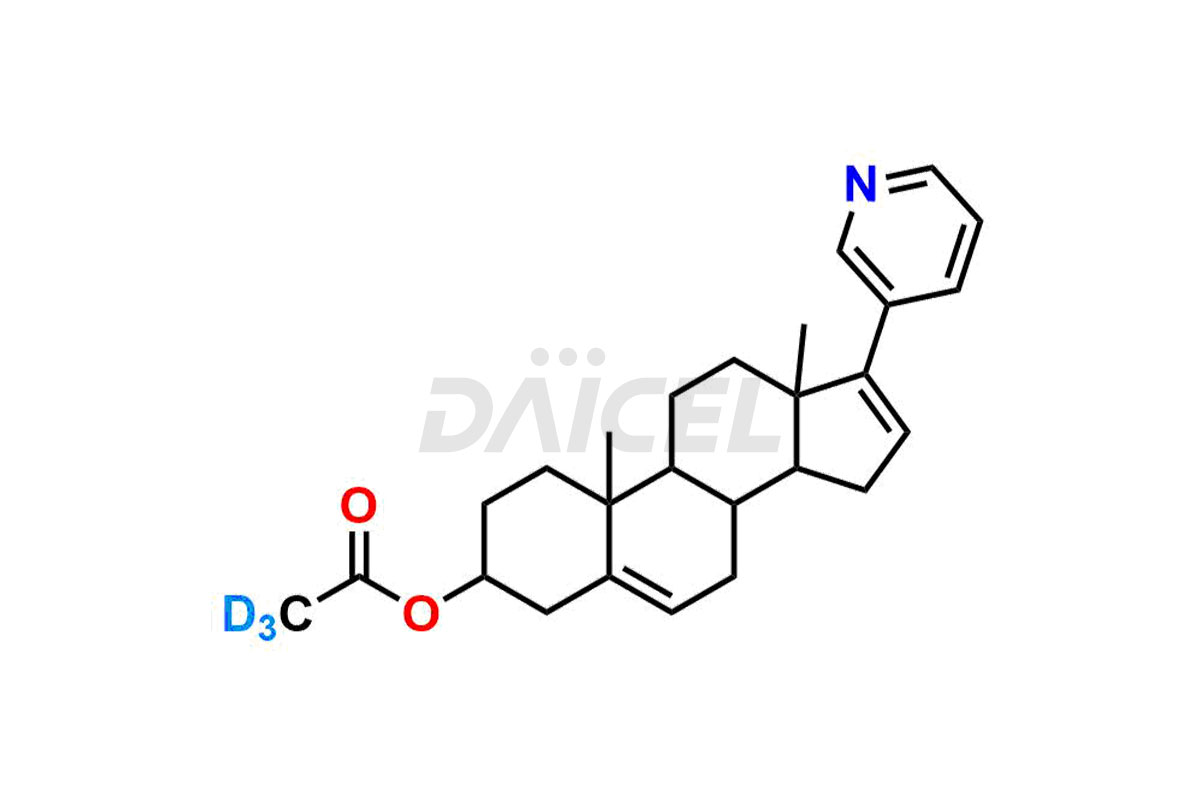
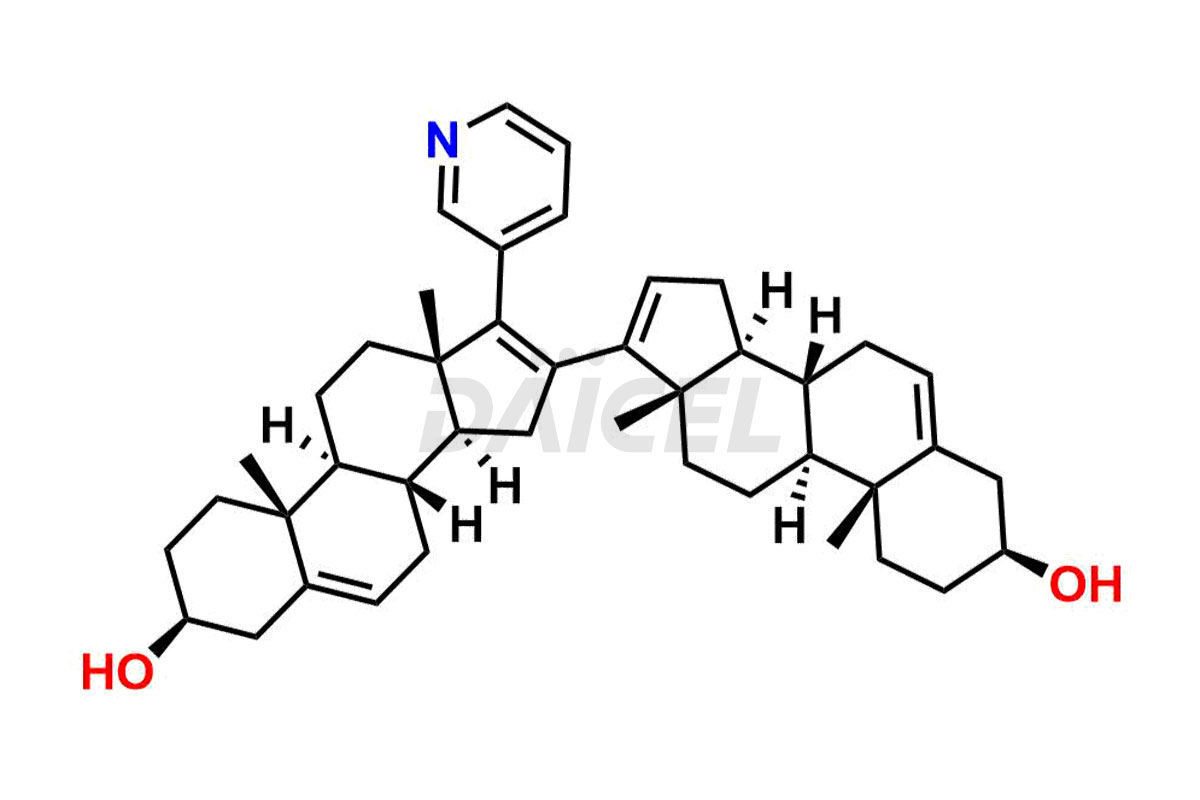
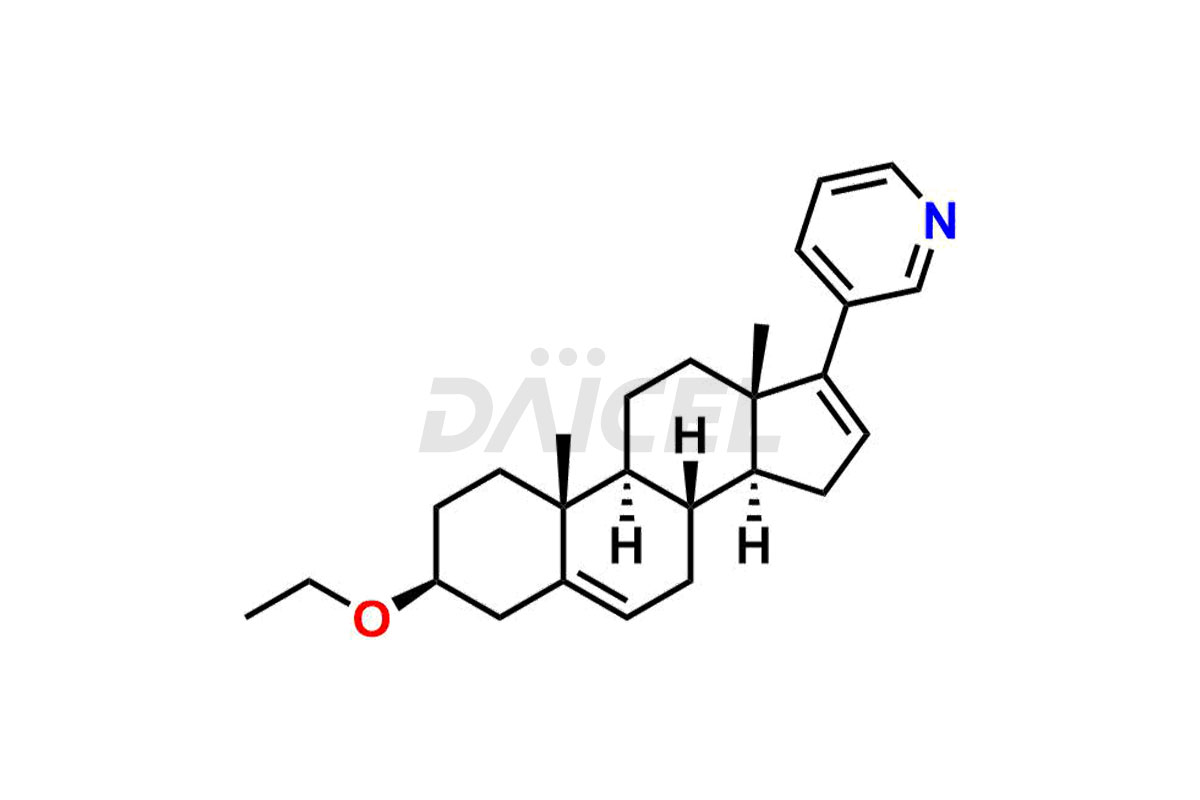
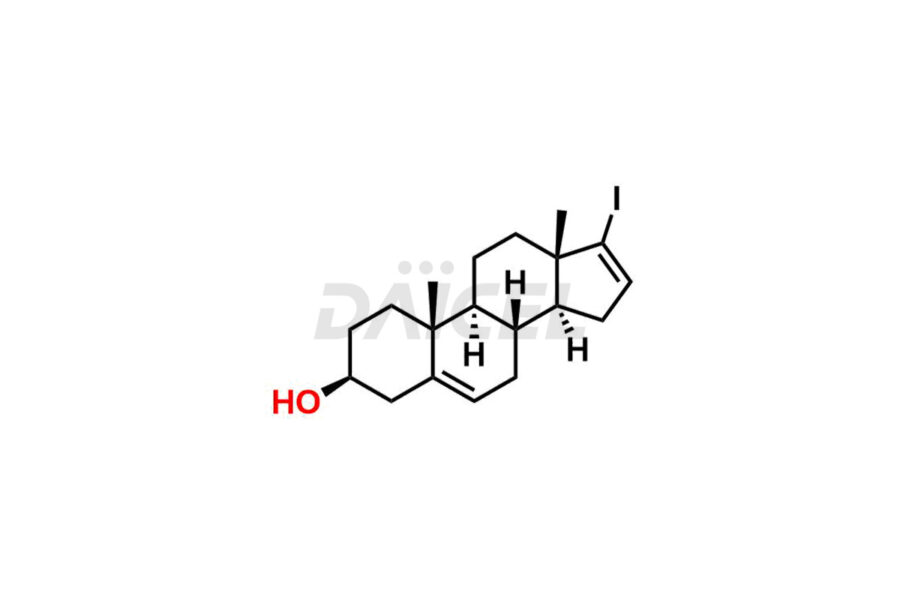
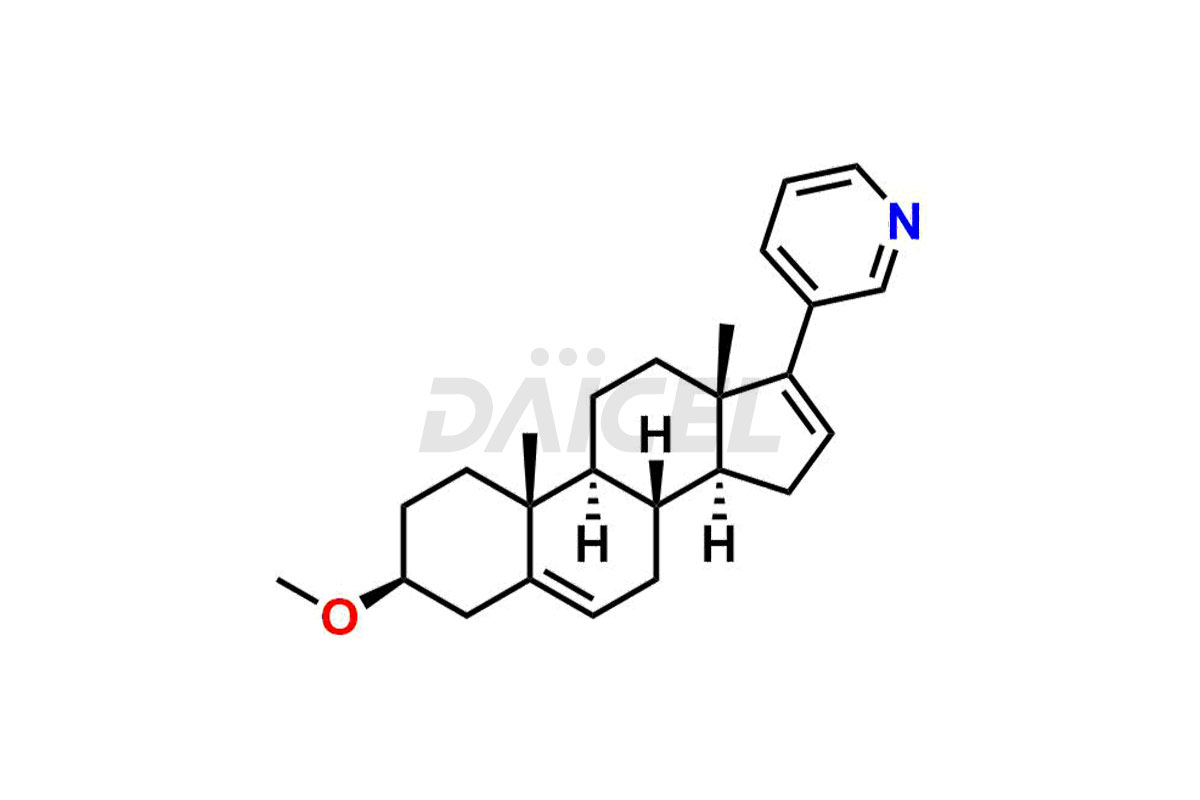
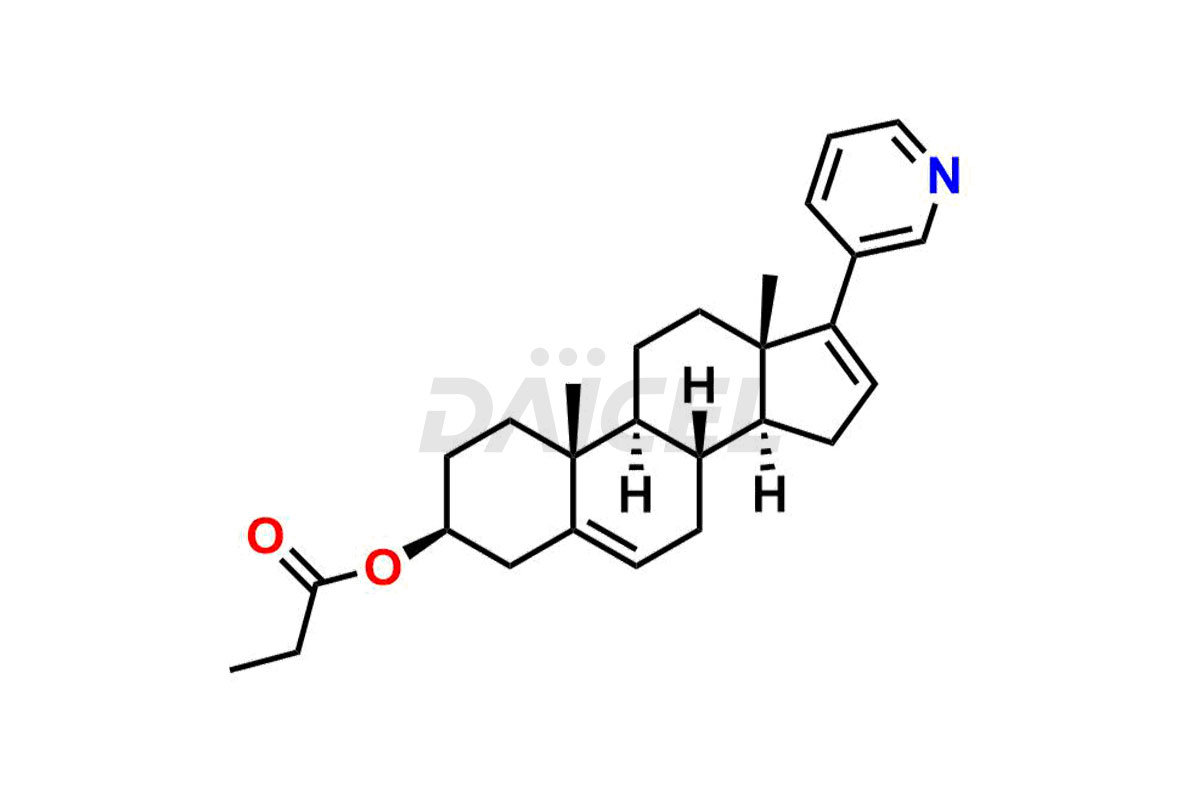
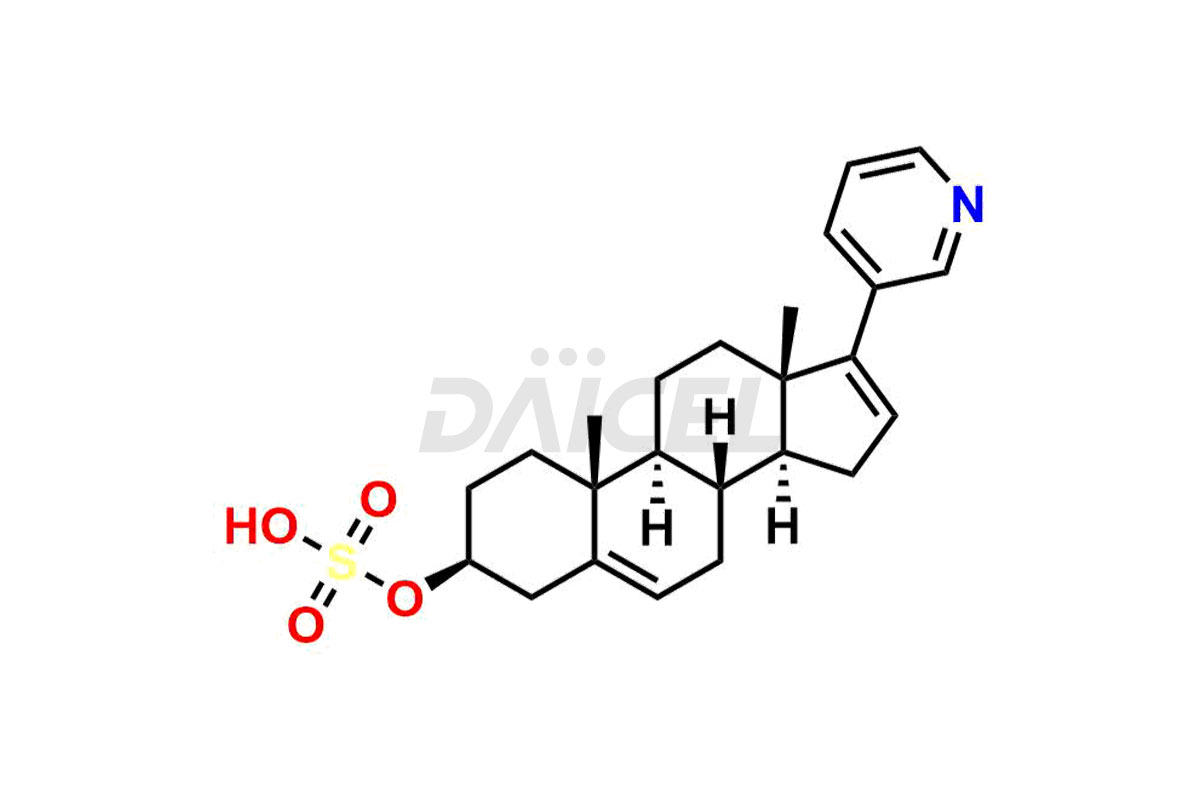
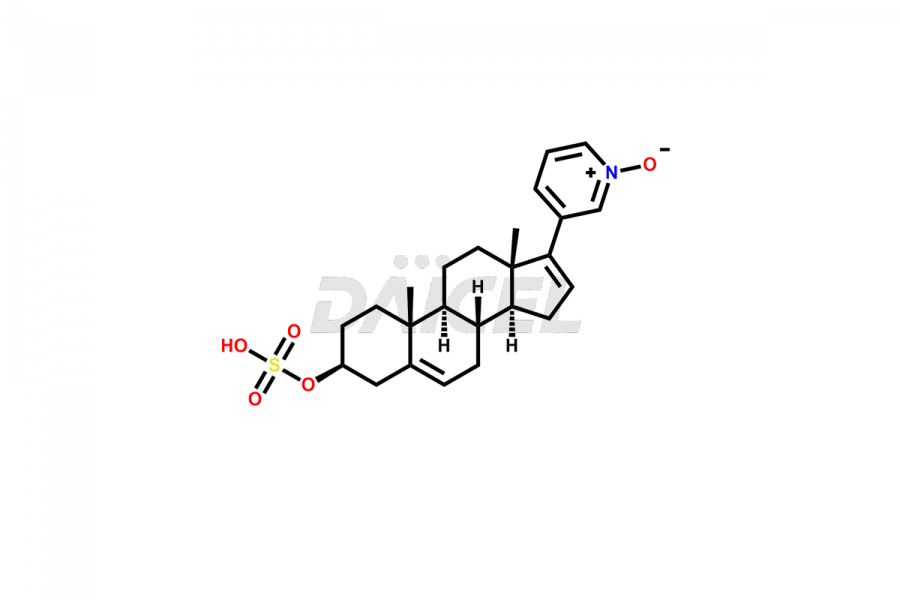
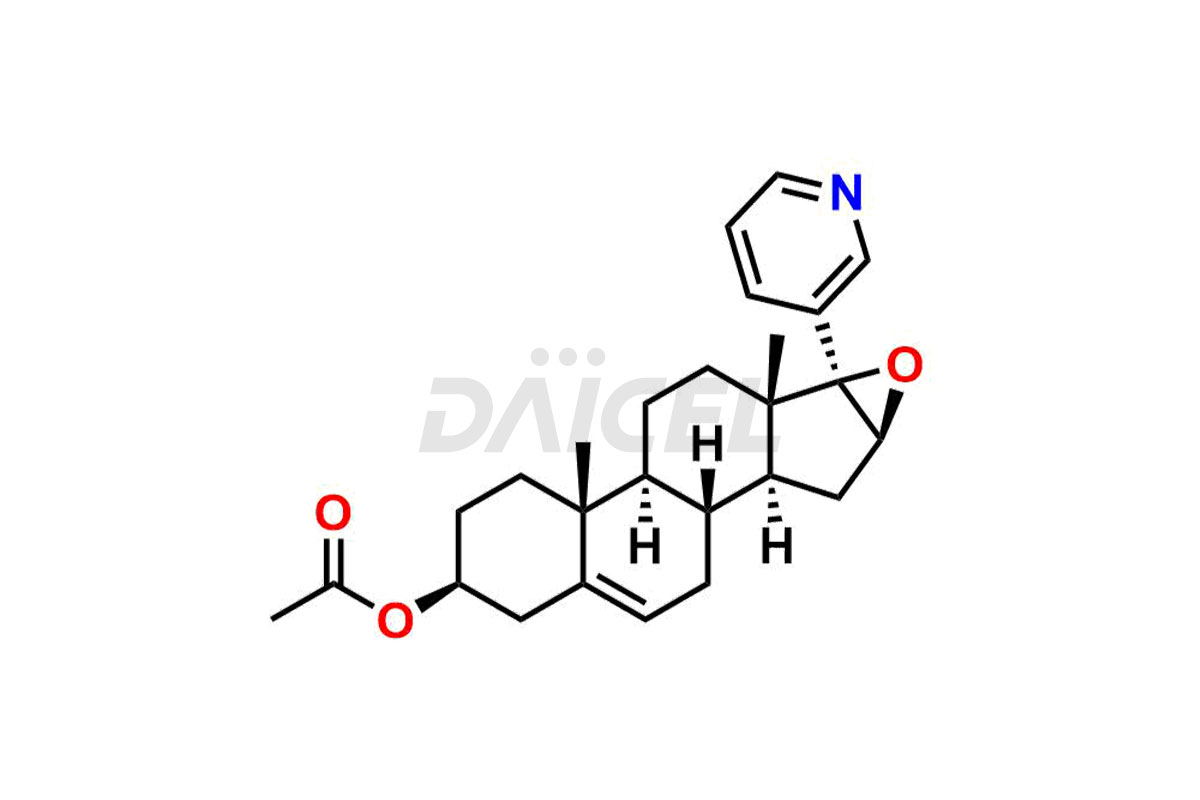
Abiraterone
General Information
Abiraterone Impurities and Abiraterone
Daicel Pharma synthesizes high-quality Abiraterone impurities like Abiraterone Acetate Impurity, Abiraterone Dimer Impurity, Abiraterone Ethyl Ether, Abiraterone Methyl Ether, Abiraterone propionate Impurity, Abiraterone Sulfate, and more, which are crucial in the analysis of the quality, stability, and biological safety of the active pharmaceutical ingredient Abiraterone. Moreover, Daicel Pharma offers custom synthesis of Abiraterone impurities and delivers them globally.
Abiraterone [CAS: 154229-19-3] is a steroidal compound having antiandrogenic properties. Abiraterone is a medicine that treats metastatic prostate cancer in men who have undergone castration. It inhibits the activity of steroid 17 alpha-monooxygenase, also known as CYP17A1. This enzyme is a member of the cytochrome p450 family and helps in testosterone synthesis.
Abiraterone: Use and Commercial Availability
Abiraterone, available under the trade name Zytiga, was the first drug to work through its mechanism. It is an irreversible inhibitor of the enzyme CYP17A1, crucial for androgens and estrogens preparation in both the adrenal glands and tumor tissues. Therefore, Abiraterone suppresses androgen synthesis in both of these locations. Abiraterone is available as abiraterone acetate combines with either methylprednisolone or prednisone and treats metastatic castration-resistant prostate cancer (CRPC) or metastatic high-risk castration-sensitive prostate cancer (CSPC). The other trade name of Abiraterone is Yonsa.
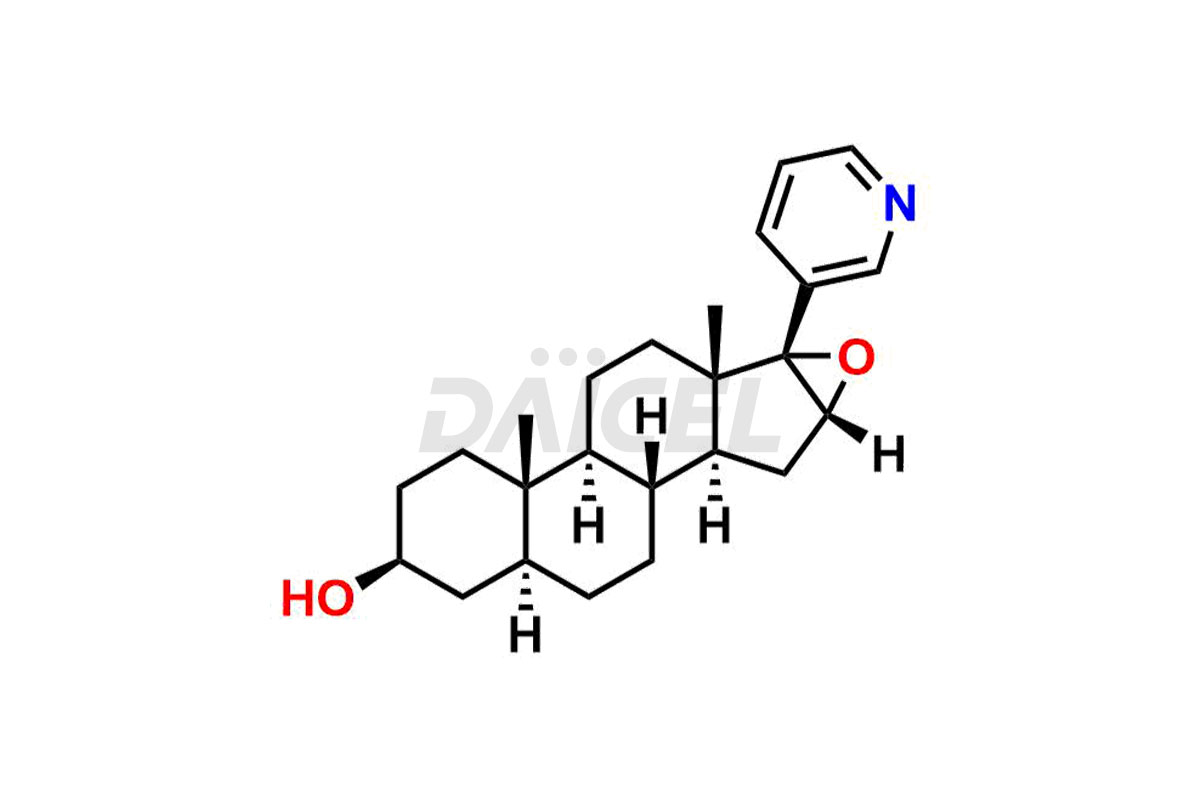
Abiraterone Structure and Mechanism of Action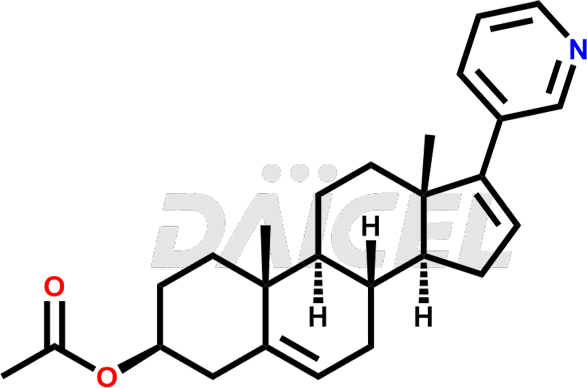
The chemical name of Abiraterone is (3β)-17-(3-Pyridinyl)androsta-5,16-dien-3-ol. Its chemical formula is C24H31NO, and its molecular weight is approximately 349.5 g/mol.
Abiraterone inhibits 17 α-hydroxylase/C17,20-lyase (CYP17) enzyme expressed in testicular, adrenal, and prostatic tumor tissues. It is for androgen biosynthesis.
Abiraterone Impurities and Synthesis
The most common Abiraterone impurities are related to the starting materials used in the synthetic process1. Impurities can form during the reaction process or as a result of the degradation of the drug substance. Controlling these impurities is vital for maintaining drug quality, safety, and efficacy.
Daicel provides a Certificate of Analysis (CoA) for Abiraterone impurity standards, including Abiraterone Acetate Impurity, Abiraterone Dimer Impurity, Abiraterone Ethyl Ether, Abiraterone Methyl Ether, Abiraterone propionate Impurity, Abiraterone Sulfate, and more. The CoA is issued from a cGMP-compliant analytical facility and contains complete characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2,3. Additional characterization data, such as 13C-DEPT and CHN, can be provided upon request. Daicel can also prepare any unknown Abiraterone impurity or degradation product and offer labeled compounds to quantify the efficacy of Abiraterone. Daicel offers Abiraterone acetate-D3, a deuterium-labeled Abiraterone compound used in bio-analytical research such as BA/BE studies. We give a complete characterization report on delivery.
References
FAQ's
References
- Barrie, Susan Elaine; Jarman, Michael; Potter, Gerard Andrew, 17-substituted steroids useful in cancer treatment, British Technology Group Ltd., United Kingdom, US5604213A, February 18, 1997
- Martins, Vanessa; Asad, Yasmin; Wilsher, Nicola; Raynaud, Florence, A validated liquid chromatographic-tandem mass spectroscopy method for the quantification of abiraterone acetate and abiraterone in human plasma, Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences, Volume: 843, Issue: 2, Pages: 262-267, 2006
- Wani, Tanveer A., Highly sensitive ultra-performance liquid chromatography-tandem mass spectrometry method for the determination of abiraterone in human plasma, Analytical Methods, Volume: 5, Issue: 15, Pages: 3693-3699, 2013
Frequently Asked Questions
Why are Abiraterone impurities a concern?
The impurities in Abiraterone affect the drug's efficacy and safety.
What are the types of Abiraterone impurities?
The types of impurities in Abiraterone are categorized into organic impurities, inorganic impurities, residual solvents, and potential genotoxic impurities.
How are Abiraterone impurities detected?
The impurities in Abiraterone were detected using analytical techniques such as high-performance liquid chromatography (HPLC) and LC–UV.
What are the temperature conditions required to store Abiraterone impurities?
Abiraterone impurities are stored at a controlled room temperature between 2-8 ⁰C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.